Is H2O a weak nucleophile?
Let’s dive into the chemistry: Nucleophilicity is the ability of a molecule or ion to donate an electron pair to an electrophile, which is a species that accepts an electron pair. In simpler terms, nucleophiles are “electron-rich” and are looking for a place to share their electrons. Hydroxide (OH-) is the conjugate base of water. It’s a stronger nucleophile than water because it carries a negative charge, making it more likely to donate its electrons.
Think of water as a person who’s content with their current relationships. They’re not actively seeking new connections. On the other hand, hydroxide is like a person who’s looking for new friends and is eager to make new connections.
This “contentment” of water stems from its structure and bonding. Oxygen, with its high electronegativity, holds the electrons in the O-H bonds more tightly than hydrogen. This leaves the oxygen atom with a partial negative charge and the hydrogen atoms with a partial positive charge. The lone pairs on the oxygen atom are also tightly held and less available to participate in reactions.
In summary: Water is a weak nucleophile due to the stability of its structure and its less-than-eagerness to donate its electron pairs. It prefers to keep things as they are. Hydroxide, being more electron-rich, is more likely to donate its electrons and thus is a stronger nucleophile.
Is water a hard nucleophile?
Think of it this way: if you have a very strong magnet, it’s going to hold onto its metal pieces tightly. Water is kind of like that magnet; it’s not going to easily let go of its electrons. In order for water to act as a nucleophile, it needs to be in a situation where it can overcome this electronegativity.
Now, “hard” nucleophiles are generally small and highly electronegative. They have a strong affinity for positively charged centers. On the other hand, “soft” nucleophiles are generally larger, less electronegative, and more polarizable. They have a preference for less positively charged centers. Water falls somewhere in the middle.
Water can act as a nucleophile, but it needs to be in the right environment. For example, if you have a very strong electrophile (something with a very positive charge), water might be able to act as a nucleophile. However, if you have something like a hydroxide ion (OH-), which is a much stronger nucleophile, then water is unlikely to be the one attacking the electrophile.
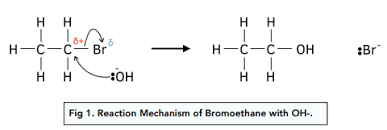
Let’s look at some examples. Water can react with an alkyl halide (like methyl bromide) to form an alcohol. In this reaction, the oxygen atom in water attacks the carbon atom in the methyl bromide, which is partially positive due to the electronegativity of the bromine atom. This is an example of water acting as a nucleophile, but it’s important to note that this reaction is only likely to occur under certain conditions.
Overall, while water is a weak nucleophile, it can still react with other molecules under the right conditions.
Let me know if you’d like more details or have other questions!
What makes a bad nucleophile?
A highly electronegative atom is less likely to share its electrons, making it a weaker nucleophile. As electronegativity increases, nucleophilicity decreases. Think of it like this: the more an atom wants to hold onto its electrons, the less likely it is to donate them to another atom, which is the essence of a nucleophile’s role.
Another factor that influences a nucleophile’s strength is its size. Bulkier nucleophiles, those with larger atoms, are less effective. This is because their size makes it more difficult for them to physically reach and attack the target molecule, also known as the substrate. Imagine trying to fit a large key into a small lock; it’s simply not going to work as smoothly.
Here’s an example: compare fluoride ion (F-) and iodide ion (I-). Fluorine is more electronegative than iodine, so fluoride is a weaker nucleophile. Additionally, iodide is a much larger atom than fluoride, which makes it a better nucleophile because it is less sterically hindered.
Steric hindrance refers to the physical obstruction that a large molecule faces when trying to approach another molecule. In the case of nucleophiles, steric hindrance reduces the likelihood of a successful attack on the substrate.
Think of it like a crowd of people: if you’re trying to get to someone in the middle of a crowded room, you’ll have a much harder time if you’re carrying a large backpack than if you’re just carrying a small purse. The same principle applies to nucleophiles; larger nucleophiles are less effective at attacking substrates due to their bulk.
So, to summarize, electronegativity and size are two key factors that determine the strength of a nucleophile. Highly electronegative atoms and bulky molecules are less likely to act as effective nucleophiles.
How is H2O a nucleophile?
Now, you might be thinking, “Hold on, water can also be an electrophile? How does that work?” Well, water can also accept electrons in certain situations. It’s like that neighbor who sometimes needs a cup of sugar (electrons). This makes it an electrophile, a species that loves to accept electrons.
Let’s dive a little deeper into why water acts as a nucleophile. Remember those lone pairs on the oxygen atom? They’re highly reactive and always looking for a chance to form a new bond. Imagine a molecule with a positive charge, like a proton (H+). This proton is like a lonely individual who’s eager to make friends. The lone pairs on water’s oxygen atom see this positive charge and think, “Hey, we can bond!” The oxygen atom readily shares its electrons with the proton, forming a new bond. This is a classic example of water acting as a nucleophile, donating its electrons to form a new bond. This process is called protonation, where water accepts a proton.
Let’s look at a more complex example. Imagine a molecule called an alkyl halide (R-X). Alkyl halides have a carbon atom attached to a halogen atom like chlorine or bromine. These halogens are quite electronegative, meaning they pull the electrons towards themselves, leaving the carbon atom slightly positive. Water, with its eager lone pairs, sees this opportunity and attacks the carbon atom, forming a new bond. The halogen atom leaves, and we get an alcohol (R-OH). In this reaction, water acts as a nucleophile by attacking the carbon atom and forming a new bond.
So, while water can act as both a nucleophile and an electrophile, its role as a nucleophile is more common because of its ability to readily donate electrons from its lone pairs.
Why is water not a good nucleophile?
Hydroxide Ion (OH-) vs. Water (H2O)
A hydroxide ion (OH-) has a negative charge concentrated on the oxygen atom, making it a strong nucleophile. This negative charge allows it to readily attack and donate its electrons to positively charged atoms.
Water (H2O), on the other hand, has two hydrogen atoms attached to the oxygen atom, which slightly pull the electron density away from the oxygen atom. This makes the oxygen atom less negatively charged and thus less attractive to positively charged atoms. Consequently, water is less reactive as a nucleophile.
The Role of Charge
The nucleophilicity of a molecule is directly related to its charge. A more negative charge means a stronger nucleophile.
Nucleophilic Substitution Reactions
Let’s consider a nucleophilic substitution reaction. In this type of reaction, a nucleophile attacks an electrophile, replacing a leaving group.
For example, consider the reaction between an alkyl halide (R-X) and a nucleophile (Nu):
R-X + Nu -> R-Nu + X
Here, the nucleophile (Nu) attacks the alkyl halide (R-X), replacing the leaving group (X).
The Importance of Nucleophilicity
In a nucleophilic substitution reaction, the speed of the reaction is dependent on the strength of the nucleophile. A stronger nucleophile will react faster, while a weaker nucleophile will react slower.
In the case of water, its weak nucleophilic character makes it a slow participant in these reactions.
In Summary
Water is a weak nucleophile because it doesn’t have a full negative charge, making it less reactive than other nucleophiles. This is why it participates slowly in nucleophilic substitution reactions.
Why is OH a stronger nucleophile than water?
Think of it this way: the hydroxide ion has a full negative charge on the oxygen, while the water molecule has a partial negative charge on the oxygen. This extra negative charge on the hydroxide ion makes it more attracted to positively charged species, which are the electrophiles. The hydroxide ion wants to share its extra electrons with a positive charge, and the water molecule is less eager to do so.
The higher electron density on the hydroxide ion also makes it more basic. A base is a substance that can accept a proton. The hydroxide ion has a greater affinity for protons than the water molecule, due to its higher electron density, making it a stronger base.
Remember, nucleophilicity and basicity are related but not the same. Both are influenced by factors like charge, electronegativity, and steric hindrance, but a strong nucleophile isn’t always a strong base, and vice versa.
Is water the strongest nucleophile?
Why? It all boils down to the fact that water is also a very good solvent. This means it’s excellent at dissolving things, including the electrophile you’re trying to react with. When water acts as a solvent, it surrounds the electrophile, making it harder for other nucleophiles to access and react with it. Think of it like a crowd of people surrounding a celebrity – it’s difficult to get close and have a conversation.
So, while water is a good nucleophile in its own right, it’s often outdone by other, stronger nucleophiles, particularly in a water-based solution.
Let’s take a look at a common example: hydroxide ions (OH-). Hydroxide ions are much more powerful nucleophiles than water molecules. This is because hydroxide ions have a negative charge, which makes them more attractive to electrophiles.
This doesn’t mean water can’t act as a nucleophile; it absolutely can. In fact, it’s often the default nucleophile in reactions that take place in water. If there isn’t a better, more powerful nucleophile around, water will step in and do the job.
It’s all about relative strength. Water is a good nucleophile, but in the presence of stronger nucleophiles, it will take a backseat.
Is water a better nucleophile than amine?
Let’s break it down. A nucleophile is a chemical species that donates an electron pair to form a new bond. The better a nucleophile is, the more readily it can donate electrons. Now, electronegativity refers to an atom’s ability to attract electrons. The more electronegative an atom, the more it wants to hold onto its electrons.
Think of it like this: Nitrogen, being slightly less electronegative, holds onto its electrons a little less tightly compared to oxygen in water. This means the nitrogen in ammonia can more easily donate its lone pair of electrons to form a new bond, making it a better nucleophile.
To further illustrate, ammonia’s lone pair is more available for attack, making it more reactive than water’s lone pairs. In a nucleophilic substitution reaction, the ammonia can easily replace a halide ion (like chloride or bromide) in a molecule, while water is less likely to do so.
The substitution reaction happens because the nitrogen atom in ammonia is better at forming a new bond with the carbon atom in the molecule, pushing out the halide ion. This is a common reaction used in organic chemistry to synthesize new molecules.
See more here: Is Water A Hard Nucleophile? | Why Is Water A Bad Nucleophile
Is water a good nucleophile?
It really depends on the specific reaction you’re looking at. Water might not be the star of the show in every reaction, but it can still play a role. For example, in some reactions, water might be involved in a faster step, while a different nucleophile is involved in the slower, rate-determining step. This means that even though water might not be the strongest nucleophile, it can still be present and contribute to the overall reaction.
Now, you might be wondering if you’ll need to worry about this in your studies. It’s true that most high school chemistry syllabuses won’t dive deep into the nuances of nucleophile strength. However, understanding this concept can give you a deeper understanding of how reactions work.
Let’s break this down a bit further. Nucleophiles are essentially “electron-rich” species that are attracted to “electron-poor” centers (called electrophiles). Water has lone pairs of electrons on its oxygen atom, making it capable of acting as a nucleophile. However, its oxygen atom is also partially positive due to the electronegativity difference between oxygen and hydrogen. This makes water a relatively weak nucleophile compared to hydroxide ion, which has a full negative charge on the oxygen atom, making it much more attractive to electrophiles.
Think of it like this: imagine you have two magnets, one is strong and one is weak. The strong magnet (hydroxide ion) will pull on the metal (electrophile) more readily than the weak magnet (water).
So, even though water might not be the best nucleophile in the room, it can still play a part in reactions. Remember, it’s all about the specific context and the other players involved in the reaction.
Is a negatively charged hydroxide ion more nucleophilic than a water molecule?
Think of it this way: A hydroxide ion has a negative charge, meaning it has an extra electron. This extra electron makes it super eager to donate to something else, like a carbon atom in a molecule. On the other hand, a water molecule is neutral, so it’s less likely to donate an electron.
This difference in charge is a big deal for nucleophilicity. Nucleophiles are like chemical “attackers” that want to grab onto positively charged atoms. The more negative a nucleophile is, the more it wants to attack and donate its extra electron. So, the hydroxide ion is a much better nucleophile because it’s got that extra negative charge.
Now, let’s get practical. Imagine we have a chloromethane molecule. It has a slightly positive carbon atom. If we add a hydroxide ion to the mix, it’s going to want to attack that positive carbon and form a new bond.
But what if we use water instead? Well, water is less negative and not as eager to attack that carbon. This means that the reaction with hydroxide ion will happen much faster, sometimes even by several orders of magnitude.
Let’s break down what this means:
Hydroxide ion is a stronger nucleophile than water, because it has a more negative charge.
Hydroxide ion will attack and react with chloromethane much faster than water because of its greater negative charge and stronger desire to donate electrons.
* This difference in reactivity makes hydroxide ion a better choice for certain chemical reactions, especially those where we need a fast and efficient reaction.
In short, the hydroxide ion is a real powerhouse of a nucleophile, making it a key player in many chemical processes.
What makes a nucleophile a base?
Here’s the breakdown:
Nucleophiles are molecules that donate electrons to form a new bond. They love to attack electron-deficient atoms, like the positively charged carbon in a carbonyl group.
Bases are molecules that accept protons (H+). They do this because they’re looking to neutralize their negative charge.
So, the same electron-richness that makes a molecule a good nucleophile also makes it a good base. It’s like they have a shared talent for electron donation. Now, it’s important to remember that not all nucleophiles are bases, and not all bases are nucleophiles. Let’s explore this further.
The “Nucleophile-Base” Overlap
While some molecules can act as both nucleophiles and bases, there are some key differences to keep in mind:
Strength: Nucleophilicity and basicity are not always directly proportional. Some molecules are good nucleophiles but weak bases, and vice versa. For example, hydroxide ion (OH-) is a strong base and a good nucleophile, but iodide ion (I-) is a weak base but a good nucleophile.
Solvent: The solvent can have a big influence on how a molecule behaves. In polar protic solvents (like water or alcohols), the nucleophilicity of a molecule is often reduced because the solvent molecules can form hydrogen bonds with the nucleophile, making it less reactive. However, in polar aprotic solvents (like acetone or DMF), nucleophiles are more reactive because the solvent molecules don’t interfere with their ability to attack electrophiles.
The Bottom Line
While the terms “nucleophile” and “base” might seem similar, there are subtle differences in their reactivity and how they interact with other molecules. Ultimately, their electron-rich nature is what unites them.
What is a Neutral nucleophilic reaction?
Think of a nucleophile as a bit like a friendly atom or molecule that’s always looking to share its electrons. It’s attracted to anything with a positive charge. In a nucleophilic substitution reaction, the nucleophile replaces another atom or group of atoms attached to the positively charged molecule. This is kind of like a swap, where the nucleophile takes the spot of the original group.
Nucleophilic addition reactions, on the other hand, are like adding to a party. The nucleophile attaches itself to the positively charged molecule without displacing anything else. It just joins the party, essentially adding to the molecule’s structure.
Now, when we talk about neutral nucleophilic reactions happening in solvents like alcohols or water, we use a special term: solvolysis. It’s a fancy way of saying that the solvent itself is acting as a nucleophile and participating in the reaction.
Nucleophilicity is a measure of how readily a molecule can act as a nucleophile, how willing it is to share its electrons. Interestingly, nucleophilicity is closely related to basicity. Basically, stronger bases tend to be better nucleophiles.
Let me give you a quick example to illustrate this. Imagine you have a molecule with a positively charged carbon atom. This carbon atom is like a magnet, attracting negatively charged species, like nucleophiles. Now, if you introduce a hydroxide ion (OH-) which is a strong base, it will readily react with the positively charged carbon atom, acting as a nucleophile. The hydroxide ion will replace another group attached to the carbon, resulting in a nucleophilic substitution reaction.
So, neutral nucleophilic reactions are basically like a dance where electrons are shared, bonds are formed, and molecules change. It’s a dynamic and fascinating process that plays a crucial role in many chemical reactions.
See more new information: linksofstrathaven.com
Why Is Water A Bad Nucleophile? The Surprising Answer
You see, nucleophiles are like chemical ninjas, sneaking around and attacking positively charged centers in molecules. They’re all about sharing electrons, and the better they are at it, the more nucleophilic they are. Now, water, being a polar molecule, has a slightly negative end (oxygen) and a slightly positive end (hydrogens). This polarity makes it seem like it should be a great nucleophile, right?
But hold on, there’s a twist! Water’s nucleophilicity is actually quite limited, and here’s why.
Water’s Hydrogen Bonding: A Double-Edged Sword
First, let’s talk about hydrogen bonding. Water molecules are masters of hydrogen bonding. They love clinging to each other, creating a network of interconnected molecules. This strong hydrogen bonding makes it hard for water to break free and attack other molecules. It’s like water is too busy socializing to go on a chemical attack!
Water’s Limited Availability: Solo Acts Don’t Always Work
Another factor is the availability of the lone pairs on the oxygen atom. These lone pairs are the key to nucleophilic attacks, but they’re also involved in hydrogen bonding. Since water is already engaged in hydrogen bonding, its lone pairs are less readily available for attacking other molecules. It’s like having a great weapon but not being able to use it because it’s tied up.
Protic Solvents: Water’s Competition
Let’s not forget about water’s role as a protic solvent. This means water readily donates protons (H+) to other molecules. This proton donation can actually hinder nucleophilic attacks by making the nucleophile less reactive. Imagine water taking away the attacking power of a nucleophile. Not cool, right?
Water’s Limited Reactivity: A Gentle Giant
So, while water is a good solvent, its nucleophilicity is hindered by its strong hydrogen bonding, its limited availability of lone pairs, and its tendency to donate protons. It’s a bit like a gentle giant. It’s powerful but doesn’t always have the aggression needed for a full-on chemical attack.
Why Do We Care?
Knowing why water is a poor nucleophile is important in various chemical reactions. For example, in organic chemistry, we often use water as a solvent but need to choose other nucleophiles to drive specific reactions.
FAQs
Q: Can water ever act as a nucleophile?
A: Yes, water can act as a nucleophile, but it’s generally weak compared to other nucleophiles.
Q: What are some examples of better nucleophiles than water?
A: Stronger nucleophiles include hydroxide ions (OH-), alkoxide ions (RO-), and amines (RNH2).
Q: How can we enhance water’s nucleophilicity?
A: We can enhance water’s nucleophilicity by increasing its concentration or by using a less protic solvent.
Q: What are the consequences of water’s limited nucleophilicity?
A: Water’s limited nucleophilicity can slow down certain reactions, making them less efficient.
Q: Are there any exceptions to water being a bad nucleophile?
A: Yes, there are exceptions. Water can be a good nucleophile in specific cases, such as in acid-catalyzed reactions.
Remember, the world of chemistry is full of fascinating nuances. Understanding why water isn’t the ultimate nucleophile helps us appreciate its unique properties and how it plays a role in various chemical processes.
nucleophilic substitution – halogenoalkanes and water – chemguide
The nucleophilic substitution is very slow because water isn’t a very good nucleophile. It lacks the full negative charge of, say, a hydroxide ion. The second step of the reaction simply tidies up the product. chemguide
Nucleophile – Chemistry LibreTexts
Notice that when oxygen is part of the hydroxide ion, it bears a negative charge, and when it is part of a water molecule, it is neutral. The O of – OH is a better Chemistry LibreTexts
7.1 Nucleophiles and Electrophiles – Chemistry LibreTexts
When thinking about nucleophiles, the first thing to recognize is that, for the most part, the same quality of ‘electron-richness’ that makes a something nucleophilic also makes it Chemistry LibreTexts
Explaining nucleophilic substitution between halogenoalkanes
Because there isn’t a full negative charge, water isn’t going to be as good a nucleophile as a negative ion like OH -, and so the reaction is slower. The chemguide
7.2: Nucleophiles – Chemistry LibreTexts
A negatively-charged hydroxide ion is much more nucleophilic (and basic) than a water molecule. In practical terms, this means that a hydroxide nucleophile will react in an Chemistry LibreTexts
8.3. Factors affecting rate of nucleophilic substitution
In pathway ‘a’, water acts as a nucleophile – this is, of course, the familiar S N 1 reaction. However, a water molecule encountering the carbocation intermediate could alternatively act as a base rather than as a Lumen Learning
What Makes A Good Nucleophile? – Master Organic
What are the factors that make a good nucleophile? For our purposes, there are at least four key factors contributing to nucleophilicity. Charge. Electronegativity. Solvent. Steric hindrance. The first two Master Organic Chemistry
Why does water favour nucleophilic substitution over elimination?
The problem is compounded by the fact that, with hydroxide as the nucleophile, an alcohol would form, which would be stabilized by hydrogen bonding Chemistry Stack Exchange
Nucleophile – Wikipedia
Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance’s nucleophilic character and is often used to compare the affinity of atoms. Neutral Wikipedia
Nucleophilicity (nucleophile strength) (video) | Khan Academy
How does F(-) become the best nucleophile in aprotic solvents and I(-) the worst? What I mean to ask is how and why exactly is the order of nucleophilicity flipped in aprotic solvents? Khan Academy
Nucleophilic Strength
Nucleophiles And Electrophiles
20.1 Explain Why The Hydroxide Ion Is A Better Nucleophile Than Water [Hl Ib Chemistry]
Nucleophile || Among Oh- And H2O Which One Is A Strong Nucleophile?
Nucleophilic Strength
Nucleophiles And Electrophiles: Crash Course Organic Chemistry #12
Nucleophiles, Electrophiles, Leaving Groups, And The Sn2 Reaction
Link to this article: why is water a bad nucleophile.
See more articles in the same category here: https://linksofstrathaven.com/how
