Would bromobenzene react SN1 or SN2?
SN2 reactions need a backside attack on the carbon with the leaving group. The bulky benzene ring makes this impossible. Imagine trying to squeeze past a wall – it’s just not going to happen!
SN1 reactions require the formation of a carbocation. However, the benzene ring stabilizes the carbon-bromine bond, making it very difficult to break. This means a carbocation is unlikely to form. Think of it like a strong glue holding the bromine tightly to the carbon.
In summary, bromobenzene doesn’t react via SN1 or SN2 because of the benzene ring’s structure. It’s like a stubborn gatekeeper, preventing any nucleophiles from getting through!
Let’s dive a little deeper into the benzene ring’s influence:
Electron Delocalization: The benzene ring has a system of delocalized electrons, which spread across the entire ring. This delocalization strengthens the carbon-bromine bond, making it harder to break. Imagine a bunch of electrons swirling around, making the bond extra strong.
Steric Hindrance: The bulky benzene ring makes it difficult for a nucleophile to approach the carbon-bromine bond from the backside, which is required for an SN2 reaction. Imagine a big, bulky object blocking the path to the bond.
So, while bromobenzene might seem like a good candidate for nucleophilic substitution, the benzene ring throws a wrench in the works! It’s all about that ring’s unique structure and properties.
Why bromobenzene does not undergo nucleophilic substitution?
Bromobenzene is a pretty stable molecule, and that stability makes it a bit of a tough nut to crack for nucleophiles.
Here’s the deal: nucleophilic substitution reactions usually involve replacing a leaving group (like bromine) with a nucleophile. There are two main types: SN1 and SN2 reactions.
SN1 reactions involve a two-step process. First, the leaving group departs, forming a carbocation. Then, the nucleophile attacks the carbocation.
Bromobenzene doesn’t play nice with SN1 reactions because the phenyl cation (the carbocation formed when bromine leaves) is super unstable. Imagine a positive charge sitting on a flat ring – not good! It’s like trying to balance a ball on a tilted plate – it just won’t stay put.
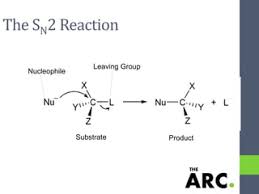
Now, let’s look at SN2 reactions. These happen in one step, where the nucleophile attacks the carbon atom attached to the leaving group from the backside. Think of it like sneaking up on someone from behind.
Bromobenzene isn’t a fan of SN2 reactions either. The benzene ring creates a lot of steric hindrance, which means there’s not enough room for a nucleophile to attack from the backside. It’s like trying to squeeze through a crowded doorway – there’s just no space!
So, the combination of a very unstable carbocation and a hindered reaction site means bromobenzene is pretty resistant to nucleophilic substitution.
To sum it up: Bromobenzene just doesn’t make things easy for nucleophiles. The phenyl cation is unstable, and the benzene ring creates a crowded environment, making it difficult for nucleophiles to attack.
Let’s delve a little deeper into the reasons behind bromobenzene’s resistance:
1. The stability of the aromatic ring: The benzene ring in bromobenzene is highly stable due to delocalization of electrons. This stability makes it difficult to break the ring’s structure, which would be necessary for nucleophilic attack.
2. The electron-withdrawing nature of the bromine atom: Bromine is an electron-withdrawing group, meaning it pulls electron density away from the benzene ring. This makes the ring even less reactive towards nucleophiles.
3. The lack of a good leaving group: While bromine is a decent leaving group, it’s not the best. In order to undergo SN1 or SN2 reactions, the leaving group needs to be able to leave easily.
4. Steric hindrance: The bulky benzene ring blocks access to the carbon atom attached to the bromine. It’s like a big, bulky guard dog protecting the carbon atom from any approaching nucleophiles.
In essence, bromobenzene is like a fortress, with its stability and crowded structure acting as barriers against nucleophiles. To overcome these challenges, specific conditions or strategies are often needed to make bromobenzene undergo nucleophilic substitution.
Why does chlorobenzene not show an SN1 or SN2 reaction?
Let’s break this down further. Resonance is a phenomenon where electrons are delocalized over multiple atoms, resulting in a more stable structure. In chlorobenzene, the lone pair of electrons on the chlorine atom can interact with the pi electron system of the benzene ring, creating a resonance structure where the carbon-chlorine bond has partial double bond character. This makes the bond shorter and stronger, resisting nucleophilic attack.
SN1 reactions involve a carbocation intermediate. The formation of a carbocation in chlorobenzene would be extremely unfavorable due to the electron-withdrawing effect of the chlorine atom. This effect further destabilizes the carbocation and hinders the SN1 reaction.
SN2 reactions require a backside attack by the nucleophile on the carbon atom. However, the steric hindrance from the benzene ring prevents the nucleophile from accessing the carbon atom, making SN2 reactions practically impossible.
In summary, the resonance in chlorobenzene strengthens the carbon-chlorine bond and makes the carbon atom less electrophilic. This hinders the formation of a carbocation intermediate required for SN1 reactions and prevents the nucleophile from attacking the carbon atom needed for SN2 reactions.
Which compound can never show SN1 as well as SN2 mechanism?
Vinyl carbocations are formed when a carbon atom with a double bond (sp2 hybridized) loses a leaving group. The positive charge is located on the sp2 hybridized carbon atom, which is directly attached to the double bond. This arrangement makes vinyl carbocations extremely unstable due to the following reasons:
Empty p-orbital: The positive charge is located on the carbon atom with an empty p-orbital, which is perpendicular to the plane of the double bond. This empty p-orbital is highly reactive and wants to gain electrons, which destabilizes the carbocation.
High electron density: The adjacent double bond contributes to the electron density around the positively charged carbon, further destabilizing the carbocation.
Steric hindrance: The sp2 hybridization and the double bond create significant steric hindrance around the carbocation, making it difficult for nucleophiles to attack.
These factors make it impossible for vinyl carbocations to participate in either SN1 or SN2 reactions. SN1 reactions require the formation of a carbocation intermediate, which is not possible for vinyl carbocations. SN2 reactions, on the other hand, involve a concerted attack by a nucleophile on the carbon atom with the leaving group. This attack is hindered in vinyl carbocations due to the steric hindrance and the unstable nature of the carbocation.
In summary, vinyl carbocations are inherently unstable and face challenges related to their structure, which makes them unable to undergo SN1 or SN2 reactions.
Why does bromobenzene not undergo SN1?
You’re right, the carbon-bromine bond in bromobenzene is pretty strong. This is because the benzene ring adds a special kind of stability, called aromaticity, to the whole structure. It’s like giving the bond a super-strength boost.
But here’s the thing: SN1 reactions need a carbocation to form first, and that’s where the trouble starts. Carbocations are positively charged carbon atoms, and they’re usually pretty unstable. They like to react quickly to get rid of that positive charge.
In bromobenzene, the benzene ring actually helps stabilize the carbon-bromine bond, making it super hard to break. Think of it as a strong wall protecting the bond. This means it’s tough to form a carbocation from bromobenzene, and that’s why SN1 reactions are pretty much out of the question.
To understand this even better, let’s imagine the benzene ring as a big, strong magnet holding onto the bromine atom. It’s holding on tight, making it really difficult for the bromine atom to leave and form a carbocation. It’s like trying to pull a magnet off a metal surface—it takes a lot of force!
So, while bromobenzene might be a strong molecule, its stability makes it a bit of a loner when it comes to SN1 reactions. The benzene ring acts like a protective shield, preventing the formation of the carbocation that’s needed for these reactions.
Is benzene SN1 or SN2?
Let’s dive a bit deeper into why benzene isn’t a good candidate for SN1 reactions. SN1 reactions involve a two-step mechanism. The first step is the formation of a carbocation, which is a positively charged carbon atom. This carbocation is highly reactive and can readily react with a nucleophile. The second step is the attack of the nucleophile on the carbocation to form the final product.
Benzene is a very stable molecule due to its delocalized pi electron system. This means that the electrons are shared equally among all the carbon atoms in the ring, making it difficult to break the bonds and form a carbocation. The stability of the aromatic ring makes it very resistant to reactions that would disrupt its structure.
Benzene is also very resistant to electrophilic attack because of its high electron density. Remember that the pi electron cloud in benzene is delocalized. This means the electrons are spread out over the entire ring, making it harder for an electrophile to find a place to attack.
While benzene doesn’t readily undergo SN1 reactions, it can participate in other reactions such as electrophilic aromatic substitution reactions. These reactions involve the attack of an electrophile on the aromatic ring, which can lead to the formation of various substituted benzene derivatives.
So, while SN1 and SN2 reactions are important in organic chemistry, they are not the primary reactions that benzene undergoes. Benzene’s unique structure and stability make it more likely to participate in other types of reactions that preserve its aromatic character.
Why does benzene not give a nucleophilic substitution reaction?
Imagine a nucleophile as a negatively charged attacker, wanting to snatch away a piece of the benzene ring. But the delocalized electron cloud is like a force field, repelling these attackers. The ring’s stability makes it resistant to changes, including nucleophilic substitution.
Now, let’s dive deeper into the mechanism of nucleophilic substitution. Typically, a nucleophile attacks an electrophilic center, like a carbon atom, leading to the displacement of a leaving group. However, in benzene, the delocalized electron cloud spreads over the entire ring, effectively distributing the electron density. This delocalization makes the carbon atoms less electrophilic, meaning they are less attractive to nucleophiles.
Think of it this way: the electrons in the benzene ring are like a bunch of friends sharing a pizza. Each carbon atom gets a little bit of the pizza, but no one gets a full slice. This sharing makes the carbon atoms less appealing to nucleophiles.
In addition, the pi electrons in the benzene ring form a stable system, which makes it difficult for nucleophiles to disrupt the structure. It’s like a tightly knit group of friends. It’s hard for someone to break into that group and disrupt their harmony.
Therefore, due to the presence of the delocalized electron cloud and the stable pi system, benzene is not easily attacked by nucleophiles, and nucleophilic substitution reactions are generally unfavorable.
What is bromobenzene substitution reaction?
Think of it like this: bromine is the electrophile, meaning it’s attracted to electrons. It wants to bond with the benzene ring. That’s where the magic happens! The bromine forms a sigma bond with the benzene ring, creating an intermediate. This intermediate is kind of like a temporary, unstable molecule.
What happens next is that a proton is removed from this intermediate. This removal leads to the formation of a substituted benzene ring, which means the bromine has now taken a permanent spot on the benzene ring, replacing a hydrogen atom.
Now, let’s dive a little deeper into the process and understand why this reaction is so important.
The electrophilic aromatic substitution reaction is a fundamental reaction in organic chemistry. It allows us to introduce new functional groups onto the benzene ring. This ability to modify the benzene ring is crucial because it allows us to create a wide variety of molecules with different properties and uses.
Think about it this way: benzene is like a blank canvas, and the electrophilic substitution reaction is our paintbrush. We can add different functional groups (like bromine, chlorine, or nitro groups) to change the properties of the canvas, creating new and interesting molecules with unique characteristics.
To get a clearer picture, let’s visualize the bromine attacking the benzene ring. The bromine molecule is polarized, meaning there’s a slight positive charge on one end and a slight negative charge on the other. This polarity allows the bromine to interact with the electron-rich benzene ring.
The positive end of the bromine molecule approaches the electron-rich pi system of the benzene ring. This interaction induces a shift in the electron density of the ring, making it more reactive. The bromine molecule then donates a positively charged bromine ion (Br+) to the benzene ring, forming a sigma bond with a carbon atom in the ring. This forms the intermediate we talked about earlier.
This intermediate is unstable because it has a positive charge on a carbon atom, which is not a stable configuration. To regain stability, a proton is removed from the intermediate by a base, such as a halide ion. This removal of the proton restores the aromatic system and results in the formation of the final product: bromobenzene.
This reaction is a fundamental building block in organic chemistry, and it’s essential for creating many valuable compounds, from pharmaceuticals to pesticides to dyes. It’s a testament to the power of organic chemistry to create new and useful molecules by manipulating the structure and properties of existing molecules.
See more here: Why Bromobenzene Does Not Undergo Nucleophilic Substitution? | Why Does Bromobenzene Not React In Sn1 Or Sn2
Why is bromobenzene unreactive?
The carbon-bromine bond in bromobenzene is stronger than in typical alkyl halides. This is due to the unique stability of the benzene ring. The electrons in the ring are delocalized, creating a cloud of electron density that spreads across all six carbons. This delocalization makes the ring very stable and resistant to change.
When we try to break the carbon-bromine bond in bromobenzene, we disrupt this electron delocalization. This makes the process less favorable and requires more energy.
Let’s look at the specific reasons:
Sn1 Reactions: In an Sn1 reaction, the first step involves the formation of a carbocation. A carbocation is a positively charged carbon atom. In bromobenzene, the carbocation that would form from the departure of bromine would be destabilized by the electron-withdrawing effect of the benzene ring. This means the benzene ring pulls electron density away from the carbocation, making it less stable.
Sn2 Reactions:Sn2 reactions are one-step processes where the nucleophile attacks the carbon atom at the same time the leaving group departs. The benzene ring in bromobenzene hinders the approach of the nucleophile. The bulky benzene ring makes it difficult for the nucleophile to reach the carbon atom bonded to the bromine.
In summary:
The strong carbon-bromine bond, the destabilizing effect on potential carbocations, and the steric hindrance of the benzene ring all contribute to bromobenzene’s lower reactivity in Sn1 and Sn2 reactions.
Remember: The special stability of the benzene ring, with its delocalized electrons, is the key player in understanding why bromobenzene is less reactive.
Why is benzyl bromide inert towards SN1 and SN2 reactions?
While benzyl bromide is classified as a primary alkyl halide, it has a unique structure that influences its reactivity. The benzyl group, which is a phenyl group attached to a CH2 group, creates an electron-rich environment around the carbon atom bonded to the bromine. This electron density helps stabilize the carbocation intermediate formed during SN1 reactions.
Now, you might be thinking, “Wait, shouldn’t a primary alkyl halide favor SN2 reactions?” You’re right in theory, but benzyl bromide doesn’t play by the usual rules. The electron-donating nature of the phenyl group actually hinders the nucleophile from attacking the carbon atom in an SN2 reaction. This is because the electrons from the phenyl ring push back on the electrons in the C-Br bond, making it more difficult for the nucleophile to come in and break that bond.
So, while benzyl bromide is technically a primary alkyl halide, its reactivity resembles that of tertiary alkyl halides in SN1 reactions and is less reactive than typical primary alkyl halides in SN2 reactions.
Let’s look at the SN1 reaction of benzyl bromide. The carbocation intermediate is stabilized by resonance with the adjacent phenyl ring. This resonance delocalization spreads the positive charge over the entire phenyl ring, making it much more stable. This stability allows the SN1 reaction to occur, even though benzyl bromide is a primary halide.
In contrast, the SN2 reaction of benzyl bromide is hindered by the electron-rich nature of the benzyl group. The phenyl ring, with its electron-donating character, makes the carbon atom bonded to the bromine less susceptible to nucleophilic attack. This steric hindrance and electron density effect, combined with the stability of the carbocation in SN1 reactions, explain why benzyl bromide prefers the SN1 pathway over the SN2 pathway.
Is 1-bromobutane an SN1 or SN2?
Let’s break down why these reactions went the way they did.
First, 1-bromobutane and 1-chlorobutane are primary halides. This means the carbon atom attached to the halogen is only bonded to one other carbon atom. Primary halides are more likely to undergo SN2 reactions because the carbon atom is less hindered, allowing the nucleophile to attack from the back side.
On the other hand, 2-bromobutane is a secondary halide. This means the carbon atom attached to the halogen is bonded to two other carbon atoms. Secondary halides are more prone to SN1 reactions because the carbon atom is more sterically hindered, making it difficult for the nucleophile to attack from the backside. Instead, the reaction proceeds through a carbocation intermediate, which is more stable for secondary halides.
2-chloro-2-methylpropane is a tertiary halide, with the carbon atom attached to the halogen bonded to three other carbon atoms. Tertiary halides are even more hindered than secondary halides, making them even more likely to react through the SN1 pathway.
Bromobenzene doesn’t react because the bromine atom is attached to a carbon atom in an aromatic ring. This makes the carbon atom very hindered and less susceptible to attack by a nucleophile.
Bromocyclohexane is a cyclic compound with a bromine atom attached to a carbon atom in the ring. The reaction of bromocyclohexane depends on the specific conditions of the reaction. Depending on the solvent, the nucleophile, and the temperature, the reaction can proceed via either an SN1 or SN2 pathway.
In summary, the type of reaction that occurs depends on the structure of the halide and the reaction conditions. Primary halides favor SN2 reactions, secondary halides favor SN1 reactions, and tertiary halides almost always react via the SN1 pathway.
See more new information: linksofstrathaven.com
Why Does Bromobenzene Not React In Sn1 Or Sn2?
Alright, so you’re wondering why bromobenzene doesn’t play nice with SN1 or SN2 reactions. It’s a pretty common question, and it’s actually a great way to dive into the world of organic chemistry. Let’s break it down.
The Nature of the Beast: Bromobenzene
First things first, let’s get to know bromobenzene. It’s an aromatic compound. That means it’s got a benzene ring, a special six-membered ring of carbon atoms with alternating single and double bonds. This ring system is super stable, and it’s going to be a major player in why bromobenzene doesn’t react in SN1 or SN2 reactions.
SN1 Reactions: The Solo Act
SN1 reactions, or substitution nucleophilic unimolecular, involve a two-step process. The first step is the formation of a carbocation, which is a positively charged carbon atom. This carbocation is highly reactive and wants to get rid of that positive charge. Then, in the second step, a nucleophile, an electron-rich species, attacks the carbocation, forming a new bond.
So, how does bromobenzene fit into this picture? Well, it’s not going to form a carbocation easily. The benzene ring, with its delocalized electrons, is incredibly stable and resistant to being disrupted. If you were to try and form a carbocation, the positive charge would be spread out over the entire ring, making it super stable and less reactive. So, it’s basically like trying to break a super strong magnet—not easy!
SN2 Reactions: The Two-Step Tango
SN2 reactions, or substitution nucleophilic bimolecular, happen in a single step. A nucleophile attacks the carbon atom attached to the leaving group, and the leaving group departs simultaneously. This is a concerted reaction, which means everything happens at the same time.
The problem with bromobenzene is that the carbon atom attached to the bromine is sp2 hybridized. It has three bonds, and these bonds are in a flat, triangular plane. For an SN2 reaction to occur, the nucleophile needs to attack from the backside of the carbon atom, which is called the backside attack. However, the flat structure of bromobenzene makes it difficult for a nucleophile to get a good angle for a backside attack. Imagine trying to kick a soccer ball from the side—it’s not going to go as far or as accurately as if you were to kick it straight on.
Additional Factors: Resonance and Electron Density
It’s not just the ring structure that makes bromobenzene so resistant to these reactions. The delocalized electrons in the benzene ring also contribute to the molecule’s stability. These electrons are constantly moving around, which makes it harder for the carbon-bromine bond to be broken. This is because the electron density around the carbon atom is higher, making it less susceptible to nucleophilic attack.
So, What Can We Do?
If you want to get bromobenzene to react, you’re going to have to find a way to overcome these challenges. One strategy is to use an electrophilic aromatic substitution reaction, where a strong electrophile like nitronium ion or sulfur trioxide attacks the benzene ring. Another strategy is to use a reaction that specifically targets the bromine atom, like a Grignard reaction or a Wittig reaction.
It’s All About the Structure
The key takeaway here is that the structure of bromobenzene is the key to understanding why it doesn’t react in SN1 or SN2 reactions. The stable benzene ring, the lack of a good leaving group, and the sp2 hybridization of the carbon atom attached to the bromine all contribute to its unreactive nature.
FAQs:
1. Can bromobenzene undergo any reactions at all?
Yes! Bromobenzene can undergo a variety of reactions, like electrophilic aromatic substitution, Grignard reaction, Wittig reaction, and others. Just not SN1 or SN2!
2. What are some examples of good leaving groups?
Good leaving groups are stable species that can leave as anions. Some examples include halides like bromine and chlorine, tosylates, and mesylates.
3. What makes a molecule a good nucleophile?
A good nucleophile is an electron-rich species that has a high affinity for positively charged atoms. Examples include hydroxide ions, alkoxide ions, and amines.
4. Are there any exceptions to these rules?
You know how it goes in chemistry, there are always exceptions! Bromobenzene might react in SN1 or SN2 under extreme conditions, but in general, it’s not going to be a major player in these types of reactions.
5. What’s the big deal with SN1 and SN2?
These reactions are super important in organic chemistry. They’re involved in all sorts of processes, from the synthesis of pharmaceuticals to the development of new materials. They’re also super useful for understanding how molecules react and interact with each other.
Now, go out there and impress your friends with your newfound knowledge of bromobenzene!
Why is Bromobenzene unreactive? – BYJU’S
Solution. Bromobenzene: Bromobenze has the chemical formula C 6 H 5 Br. The resonating structures of Bromobenzene are given below: Reactivity: In most S N 1 and S N 2 reactions, bromobenzene is unreactive. It is mostly due to the extremely strong carbon-bromine BYJU’S
Why is Bromobenzene unreactive? – Vedantu
Bromobenzene is unreactive mostly in Sn1 and Sn2 reactions. This is due to the fact that the carbon bromine bond present in the reaction is very strong as Vedantu
why is bromobenzene unreactive in sn1 and sn2 | Question AI
Bromobenzene is unreactive in both SN1 and SN2 reactions due to its unique structure and chemical properties. The presence of a strong carbon-benzene bond makes it questionai.com
Why does bromobenzene not react in both SN1 and SN2
Bromobenzene does not undergo SN1 or SN2 reactions readily due to its resonance stabilization. The delocalized electrons on the benzene ring are part of its Brainly
Why bromobenzene does not react in both SN1 and SN2 reactions?
Bromobenzene does not react in both SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular) reactions because of its Brainly
Why is bromobenzene unreactive in both Sn1 and Sn2 conditions?
Bromobenzene is unreactive in both SN1 and SN2 conditions due to its stable aromatic ring, which hinders nucleophilic attack and doesn’t support Brainly
How do you determine if a reaction will be – Socratic
Kinetics:- The rate of the SN1 reaction is proportional to the concentration of the alkyl halide but not the concentration of the nucleophile. It follows a first-order rate Socratic
Bromobenzene does not react via sn1 or sn2 pathway
Bromobenzene does not react via SN1 or SN2 pathway because the structure of the ring does not allow for a backside attack in the case of SN2 or the formation of a carbocation in SN1. Bromocyclohexane on the Course Hero
SOLVED: You will probably find that bromobenzene either
VIDEO ANSWER:Hello students welcome here in this question. If K two is greater than kevin then the overall reaction is Sn one are Sm two. Okay, explain why the question is Numerade
Answered: How does bromobenzene react differently… | bartleby
Arrange the Alkyl chlorides in the order of reactivity in SN2 reaction according to the data you obtained and compare it with theoretical order of reactivity. Are they the same? If bartleby.com
Which Reaction Faster? Sn1 Or Sn2?
Class 12- Haloalkane || Nucleophilic Substitution Reaction Example || Sn1 \U0026 Sn2 || Order Of Sn1 Sn2
Choosing Between Sn1/Sn2/E1/E2 Mechanisms
Sn2 Sn1 E1 E2 Reaction Mechanisms Made Easy!
Nucleophilic Substitution Reactions – Sn1 And Sn2 Mechanism, Organic Chemistry
Nucleophilic Substitution Reactions | Sn1 Reaction And Sn2 Reaction
Sn2 Intramolecular Reactions
Link to this article: why does bromobenzene not react in sn1 or sn2.
See more articles in the same category here: https://linksofstrathaven.com/how