How to identify an allylic halide?
Halides, like fluorine, chlorine, bromine, and iodine, are super important in chemistry. Allylic halides are special types of halides that have a unique structure. Think of it like this: imagine a chain of carbon atoms. If the last carbon atom in the chain is directly attached to a carbon atom with a double bond, and this last carbon atom also has a halogen attached to it, then you’ve got yourself an allylic halide!
Let’s break down that definition a bit more.
Chain of carbon atoms: This refers to a series of carbon atoms connected to each other in a row.
Double bond: This is a special kind of bond between two carbon atoms where they share two pairs of electrons. Think of it as a stronger connection between them.
Last carbon atom in the chain: This is the carbon atom at the very end of the chain.
Halogen: This is one of those elements we talked about earlier: fluorine, chlorine, bromine, or iodine.
So, to put it simply, an allylic halide has a halogen attached to a carbon atom that’s right next to a carbon atom with a double bond.
For example, if you have a molecule with a chain of three carbon atoms and the last carbon atom is attached to a bromine atom and the middle carbon atom has a double bond with the first carbon atom, you have an allylic halide.
Think of the allylic position like a special spot on a molecule. It’s like the “sweet spot” where a lot of interesting chemical reactions can happen. This is because the double bond and the halogen next to it create a special type of instability that makes the molecule more reactive.
Here are a few more examples to help you get the hang of it:
3-chloropropene: This molecule has a chain of three carbon atoms. The last carbon atom has a chlorine atom attached to it. The middle carbon atom has a double bond with the first carbon atom. This makes it an allylic halide.
1-bromobut-2-ene: This molecule has a chain of four carbon atoms. The first carbon atom has a bromine atom attached to it. The second carbon atom has a double bond with the third carbon atom. This is also an allylic halide.
So, remember, an allylic halide is like a special type of molecule where a halogen is attached to a carbon atom that’s next to a carbon atom with a double bond. It’s a key feature to keep in mind when you’re studying organic chemistry!
What is an allylic halogen?
Allylic halides are organic compounds where the halogen atom is attached to a sp3 hybridized carbon atom, which is directly next to a carbon-carbon double bond (C=C).
Think of it like this: you have a double bond between two carbon atoms. The carbon atom next to that double bond is where the halogen atom is attached.
Now, let’s get a little deeper. The sp3 hybridization of the carbon atom means it forms four single bonds, one of which is to the halogen atom. This is important because it affects the reactivity of the allylic halide.
Because of this special arrangement, allylic halides exhibit unique properties and reactions. They tend to be more reactive than other alkyl halides due to the presence of the double bond, making them useful in various organic reactions. For instance, they readily undergo SN1 and SN2 reactions, leading to the formation of various organic compounds.
The presence of the double bond also influences the reactivity of the halogen atom. Due to the electron-donating nature of the double bond, the carbon-halogen bond becomes weaker, making it more susceptible to nucleophilic attack.
Let me know if you have any further questions about allylic halides. I’m happy to help!
What is the difference between allyl halide and vinyl halide?
In alkyl halides, the carbon atom holding the halogen has four single bonds. Think of it like a central carbon with four arms, each holding a different atom.
Now, in vinyl halides, the carbon atom attached to the halogen is part of a double bond with another carbon. This means that carbon only has three bonds—the double bond and a single bond to the halogen.
Allyl halides are a bit different. They have a halogen attached to a carbon that is directly adjacent to a carbon-carbon double bond. This means the carbon holding the halogen is single-bonded to the double bond, but it is not part of the double bond itself.
Let’s visualize these differences with a simple example:
Alkyl halide: Think of chloromethane (CH3Cl). The carbon is single-bonded to three hydrogen atoms and the chlorine atom.
Vinyl halide: Imagine chloroethene (CH2=CHCl). Here, the chlorine is bonded to a carbon that is part of a double bond with another carbon.
Allyl halide: Picture 3-chloropropene (CH2=CHCH2Cl). The chlorine is attached to a carbon that is adjacent to a carbon-carbon double bond.
So, remember this:
Alkyl halides: The carbon with the halogen has four single bonds.
Vinyl halides: The carbon with the halogen is part of a double bond.
Allyl halides: The carbon with the halogen is attached to a carbon that is part of a double bond, but the halogen-bearing carbon itself is not.
Understanding these structural differences is crucial as they influence the reactivity and properties of these compounds. For example, vinyl halides often exhibit a greater resistance to nucleophilic substitution reactions due to the electron-withdrawing effect of the double bond. This difference in reactivity can lead to interesting applications in organic synthesis.
What is vinyl and allyl halide?
Allyl halides have a halogen atom attached to a sp³ hybridized carbon atom directly adjacent to a carbon-carbon double bond. Think of it like a halogen atom sitting next to a “gateway” to a double bond. This unique structure gives them distinct reactivity.
Vinyl halides, on the other hand, have a halogen atom directly attached to a sp² hybridized carbon atom within an aliphatic compound. In simpler terms, the halogen is part of the double bond itself, creating a different chemical environment.
Let’s dig a little deeper into the structure of these compounds:
Allyl halides: The carbon atom holding the halogen atom is sp³ hybridized, meaning it has four single bonds, including the one to the halogen. The double bond is on the neighboring carbon atom. This arrangement makes the halogen atom relatively reactive, readily participating in reactions like nucleophilic substitution.
Vinyl halides: The carbon atom holding the halogen atom is sp² hybridized, meaning it has three bonds, including the double bond and the bond to the halogen. This structure results in a more stable arrangement, making the halogen less reactive. However, they can undergo reactions like electrophilic addition.
Understanding the hybridization and bonding patterns of these compounds is crucial for predicting their reactivity. These structural features influence their reactions, making them valuable in organic synthesis and various industrial applications.
What is allyl halide?
These halides are special because they’re quite reactive in both SN1 and SN2 reactions. These are important reactions in organic chemistry, and allylic halides often participate in them.
Think of it this way: The double bond creates a bit of “electron density” near the halogen. This makes the halogen more likely to leave as a negative ion (a halide ion), which is what happens in SN1 and SN2 reactions.
Here’s a bit more detail:
SN1 reactions involve a two-step process. First, the halide leaves, creating a carbocation. This carbocation is then attacked by a nucleophile (an electron-rich species). Allylic halides can form stable carbocations because the double bond can “share” its electrons with the positively charged carbon, making it more stable. This makes them more likely to undergo SN1 reactions.
SN2 reactions are one-step processes where a nucleophile directly attacks the carbon atom attached to the halogen, pushing the halide out. The double bond in allylic halides makes the carbon atom more accessible for attack by a nucleophile, making them more likely to undergo SN2 reactions.
So, allylic halides are interesting molecules that are particularly reactive in common organic chemistry reactions due to the special position of the halogen atom in relation to the double bond. This reactivity makes them useful building blocks for creating new and complex organic molecules.
Which is not an allylic halide?
An allylic halide is a special type of organic compound where a halogen atom (like bromine) is attached to a carbon atom adjacent to a carbon-carbon double bond. This positioning gives allylic halides some unique reactivity, making them important players in organic chemistry reactions.
Now, let’s look at 4-Bromobut-1-ene. The bromine atom is attached to the fourth carbon atom in the chain, which is three carbons away from the double bond. Since the bromine isn’t directly connected to a carbon adjacent to the double bond, 4-Bromobut-1-ene doesn’t fit the definition of an allylic halide.
To illustrate this further, let’s take a look at a few examples of allylic halides:
3-Chloroprop-1-ene: Here, the chlorine atom is attached to the third carbon, which is directly next to the double bond.
1-Bromo-2-methylprop-2-ene: The bromine atom is on the first carbon, which is adjacent to the double bond.
In contrast, 4-Bromobut-1-ene is considered a primary alkyl halide because the bromine is attached to a primary carbon (a carbon atom bonded to only one other carbon atom).
Understanding the distinction between allylic halides and other types of halides is crucial for correctly predicting their reactivity and identifying potential products in organic reactions.
How do you identify allylic?
Think of it this way: an allylic carbon is always next door to a double bond. It’s bonded to a carbon atom which is doubly bonded to another carbon atom.
You can picture this with the general formula: R-CH2-CH=CH2. The carbon marked with an asterisk is the allylic carbon.
One key thing to remember is that allylic carbons are sp3 hybridized. This means they have four single bonds, which makes them different from vinyl groups, which are sp2 hybridized.
Now, let’s dive a little deeper into why this distinction matters. The sp3 hybridization of the allylic carbon makes it more reactive than a regular carbon. This is because the allylic carbon’s electron density is spread out a bit, making it more susceptible to attack by electrophiles.
Here’s a visual analogy: imagine the allylic carbon like a sponge. It’s porous, meaning it can easily absorb things. In this case, the “things” are electrophiles. The vinyl carbon, on the other hand, is like a solid block. It’s less porous and less likely to react with electrophiles.
Understanding the allylic carbon’s unique structure and reactivity is crucial for comprehending organic reactions. It helps us predict how molecules will behave and even design new reactions for synthesizing complex compounds.
See more here: What Is An Allylic Halogen? | What Is An Allyl Halide
What is an allylic halide?
Allylic halides are organic compounds where a halogen atom is attached to a sp3 hybridized carbon atom that’s directly next to a carbon-carbon double bond (C=C). Think of it like this: the halogen is sitting right next to the double bond, but not directly on it.
Let’s look at an example: CH3-CH=CH-CH2Cl. Here, the chlorine atom (Cl) is attached to a sp3 hybridized carbon (the one with the four single bonds) that’s next to the double bond. This makes it an allylic halide.
Now, let’s examine the options you provided:
(C) 1-Bromobut-2-ene
If you draw out the structure of this compound, you’ll find that the bromine atom is attached to a sp3 hybridized carbon that’s directly adjacent to the double bond. This fits the definition of an allylic halide.
(D) 4-Bromobut-1-ene
In this case, the bromine atom is attached to a carbon atom that is three carbons away from the double bond. This doesn’t meet the requirement of being directly next to the double bond. Therefore, this compound is *not* an allylic halide.
Understanding Allylic Halides
So, what’s so special about allylic halides? They exhibit unique reactivity due to their structure. This reactivity stems from the close proximity of the halogen atom to the double bond. This allows for a variety of chemical reactions, often leading to the formation of new carbon-carbon bonds.
One important reaction is allylic substitution. This is where the halogen atom is replaced by another group, like a nucleophile. This substitution reaction can occur via SN1 or SN2 mechanisms depending on the specific conditions and the nature of the allylic halide.
Another important aspect of allylic halides is their role in polymerization. They can act as initiators in free radical polymerization, a process where polymer chains are built up by adding monomers to a growing chain.
In summary, allylic halides are a special class of organic compounds with unique reactivity due to their proximity to a double bond. Their versatility makes them crucial in various chemical reactions and synthetic applications.
What is the functional group of alkyl halides?
Now, something interesting happens because of this bond. The halogens, except for iodine, have a stronger pull on electrons than carbon does. This means they are more electronegative. This difference in electronegativity is what makes the carbon-halogen bond so special.
Because of this difference, the bond has a slight polarity. The halogen atom is slightly negative, and the carbon atom is slightly positive. This polarity is important for the reactivity of alkyl halides, as it makes the carbon atom more susceptible to attack by nucleophiles.
Nucleophiles are species that are attracted to positive charges, and they will readily react with the carbon atom in the carbon-halogen bond. This reaction is a fundamental reaction in organic chemistry, and it forms the basis for many important transformations.
To give you a more concrete example, let’s think about the reaction of an alkyl halide with a hydroxide ion (OH-). This hydroxide ion is a strong nucleophile and will readily attack the carbon atom in the carbon-halogen bond. The result of this reaction is the formation of an alcohol and a halide ion. This type of reaction is known as a nucleophilic substitution reaction.
Understanding the carbon-halogen bond and its polarity is key to understanding the reactivity of alkyl halides. It opens the door to a world of fascinating chemical transformations!
Are allylic halides a good electrophile for bimolecular nucleophilic substitution reactions?
Let’s break down why allylic halides are good electrophiles for S N 2 reactions:
Electrophilicity: The carbon atom attached to the halogen or tosylate group in an allylic halide is more electrophilic than a typical alkyl halide. This is because the adjacent double bond helps to stabilize the developing positive charge on the carbon atom in the transition state.
Steric Hindrance: Allylic halides are less sterically hindered than tertiary halides, which makes them more susceptible to nucleophilic attack. This is because the double bond helps to hold the bulky substituents away from the reaction site.
Reactivity: The S N 2 reaction is favored by the presence of a good leaving group and a strong nucleophile. Allylic halides have good leaving groups (halides and tosylates) and react readily with strong nucleophiles like hydroxide, alkoxide, and cyanide ions.
In summary, allylic halides are good electrophiles for S N 2 reactions due to their increased electrophilicity, reduced steric hindrance, and the presence of good leaving groups. These factors combine to make allylic halides highly reactive towards nucleophilic attack. This makes them versatile starting materials for a wide range of organic reactions, including the synthesis of complex molecules.
See more new information: linksofstrathaven.com
What Is An Allyl Halide: Definition, Properties, And Reactions
What is an Allyl Halide?
An allyl halide is an organic compound that contains a halogen atom directly attached to a carbon atom next to a carbon-carbon double bond. The allyl group is a functional group in organic chemistry, meaning it’s a specific arrangement of atoms within a molecule that gives it distinct chemical behavior.
Think of it like this: Imagine a chain of three carbon atoms. The first and second carbon atoms are connected by a double bond, and the third carbon atom has a halogen atom attached to it. This is the basic structure of an allyl halide.
Why are Allyl Halides Important?
These compounds are pretty important in organic chemistry. They are versatile building blocks for synthesizing a variety of other organic compounds. You can find them in a lot of different applications, like:
Polymer chemistry: They’re used to make polymers, which are long chains of molecules that have lots of uses, from plastics to rubber.
Pharmaceuticals: Many medicines and drugs contain allyl halides.
Pesticides: Some pesticides and herbicides contain allyl halides.
Industrial chemicals: They’re also used as starting materials in the production of other chemicals used in industry.
The Properties of Allyl Halides
Allyl halides have some interesting properties that make them useful in different ways:
Reactivity: They are quite reactive due to the presence of the double bond. This double bond makes them susceptible to various chemical reactions, which is why they are good starting materials for many synthetic reactions.
Stability: They are also relatively stable, which means they can be stored and handled without decomposing too quickly.
Polarity: They have a bit of polarity, which is a separation of charge within the molecule. This polarity helps them to interact with other molecules, making them good solvents or reagents for certain reactions.
The Different Types of Allyl Halides
Allyl halides can be classified based on the halogen atom attached to them. The most common types include:
Allyl chloride: It has a chlorine atom attached to the allyl group.
Allyl bromide: It has a bromine atom attached to the allyl group.
Allyl iodide: It has an iodine atom attached to the allyl group.
Naming Allyl Halides
Naming these compounds is pretty straightforward. You just need to follow the basic IUPAC rules for naming organic compounds:
1. Identify the longest carbon chain: Find the longest continuous chain of carbon atoms that includes the double bond and the halogen atom.
2. Number the carbon atoms: Start numbering the carbon atoms from the end of the chain closest to the double bond.
3. Locate the halogen: Indicate the position of the halogen atom by its number.
4. Add the suffix “halide”: Replace the “e” in the alkane name with “halide” to indicate the halogen atom.
For example, 3-chloro-1-propene is an allyl halide with chlorine at the third carbon atom and a double bond between the first and second carbon atoms.
Reactions of Allyl Halides
Allyl halides participate in a variety of reactions, both in the lab and in nature. Here are some of the most important reactions:
Nucleophilic substitution reactions: The double bond in allyl halides makes them good targets for nucleophiles. These are molecules or ions that have a negative charge and are attracted to positively charged atoms. The nucleophile attacks the carbon atom attached to the halogen atom, displacing the halogen and forming a new bond. This is called nucleophilic substitution.
Addition reactions: Another important reaction of allyl halides is addition reactions. In these reactions, a molecule adds across the double bond, breaking the double bond and forming a new single bond.
Elimination reactions: Allyl halides can also undergo elimination reactions. This is where a small molecule, like a hydrogen halide, is removed from the molecule, forming a double bond in a different location.
Applications of Allyl Halides
Now, let’s look at how these compounds are used in different areas:
1. Polymer Chemistry
Allyl halides play a crucial role in polymer chemistry. They are used to make various polymers, which are large molecules made up of repeating units.
Polymers are used in countless applications, including plastics, rubber, and adhesives.
* Allyl halides are particularly useful for making polymers that are resistant to heat, chemicals, and weathering.
2. Pharmaceuticals
Many allyl halides are found in pharmaceuticals and drugs. They have a wide range of biological activities, making them valuable in medicinal chemistry.
Antibiotics: Some allyl halides are used to fight bacterial infections.
Anti-inflammatory drugs: They are also used in drugs that reduce inflammation.
Anticancer drugs: Allyl halides are being investigated for their potential use in cancer treatments.
3. Pesticides and Herbicides
Certain allyl halides are used in pesticides and herbicides. They are effective in controlling weeds and pests but must be used cautiously due to potential environmental concerns.
4. Industrial Chemicals
Allyl halides serve as starting materials for manufacturing other industrial chemicals. These chemicals are used in many different industries, including manufacturing, construction, and agriculture.
FAQs
Q: Are allyl halides harmful?
A: Some allyl halides can be harmful, especially in high concentrations. They can irritate the skin, eyes, and respiratory system. It’s important to handle them with caution and follow safety guidelines.
Q: How are allyl halides made?
A:Allyl halides are typically synthesized from allyl alcohols by reacting them with halogens like chlorine, bromine, or iodine in the presence of a suitable catalyst.
Q: What are some examples of allyl halides?
A: Some common examples include:
Allyl chloride (CH2=CHCH2Cl)
Allyl bromide (CH2=CHCH2Br)
Allyl iodide (CH2=CHCH2I)
3-chloro-1-propene
Allyl fluoride (CH2=CHCH2F)
Q: What are the environmental impacts of allyl halides?
A: Some allyl halides can contribute to air and water pollution, so it’s important to use them responsibly and dispose of them properly.
Q: What are the future applications of allyl halides?
A: Researchers are exploring new applications for allyl halides in areas like:
Nanomaterials: Allyl halides can be used to synthesize new nanomaterials with unique properties.
Biomaterials: Allyl halides are being investigated for their potential use in biomaterials for tissue engineering and drug delivery.
So there you have it! That’s a basic rundown of allyl halides. I hope this explanation has cleared up any confusion and provided you with some valuable information.
Allylic Halide – Chemistry LibreTexts
An allylic halide is an alkyl halide in whose molecule there are one or more halogen atoms on allylic carbons. eg: Gamini Gunawardena from the OChemPal site (Utah Valley University) Chemistry LibreTexts
Introduction to Alkyl Halides – Chemistry Steps
Alkyl halides are molecules in which a halogen is bonded to an sp 3 hybridized carbon atom. They are classified into Primary, Secondary, and Tertiary Chemistry Steps
Alkyl Halides: Definition, Structure, Examples, and Reactions
Alkyl halides or haloalkanes are hydrocarbons that incorporate halogen elements (fluorine, chlorine, bromine, or iodine) into their molecular structure. The halogen atom attaches to Chemistry Learner
14.4: Alkyl Halides – Chemistry LibreTexts
Allylic (2-Propenyl) Halides. Halogen compounds in which the carbon-halogen bond is adjacent to a double bon, as in are known as allylic halides. The simplest example is 3-chloropropene, Chemistry LibreTexts
10.1 Names and Structures of Alkyl Halides – OpenStax
This polarity results in a dipole moment for all the halomethanes and implies that the alkyl halide C−X carbon atom should behave as an electrophile in polar reactions. We’ll soon OpenStax
Alkyl halide nomenclature and classification – Khan Academy
So ethyl chloride is an example of a primary alkyl halide. If you look at isopropyl chloride down here. This is the carbon that’s bonded to our halogen and that carbon is bonded to two Khan Academy
Alkyl Halide – Definition, Classification, Examples,
Alkyl halides or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic BYJU’S
Allyl halide – Wikipedia
Allyl halides are organic halides containing an allyl group . Allyl halides include: Allyl chloride. Allyl bromide. Allyl iodide. Allyl fluoride [Wikidata] Wikipedia
8.7: Benzylic Halides, Allylic Halides, Vinylic Halides, and Aryl …
The following table summarizes the expected outcome of alkyl halide reactions with nucleophiles. It is assumed that the alkyl halides have one or more beta-hydrogens, Chemistry LibreTexts
What Is Vinylic || Allylic || Benzylic || Aryl Halide|| Organic Chemistry
Allylic And Benzylic Halides – Sn1 And Sn2 Reactions
Allyl Alcohol And Allyl Bromide From Pineapple Perfume
Use Of Allyl,Vinyl,Acryl,Aryl (Iupac) – Iit Jee \U0026 Neet Organic | Vineet Khatri Sir | Atp Star Kota
What Are Allylic And Benzylic Halides | How To Identify Allylic And Benzylic Halides
Common Names: Allyl, Vinyl, Benzyl, Phenyl | Organic Chemistry
Aryl Halides And Vinylic Halides – Sn1 And Sn2 Reactions
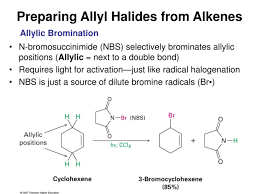
Link to this article: what is an allyl halide.
See more articles in the same category here: https://linksofstrathaven.com/how
