Under what conditions are molarity and molality values essentially equal?
Why? Think of it this way: in a dilute solution, the solvent (water, in this case) dominates the volume. The solute is present in a much smaller amount. So, the volume of the solution is almost entirely due to the solvent. In molality, we express the concentration as moles of solute per kilogram of solvent. Since the solvent’s mass doesn’t change much with temperature or pressure, molality remains relatively constant.
In very dilute solutions, the small amount of solute barely impacts the overall volume. Therefore, the volume of the solution is essentially equal to the volume of the solvent. This makes the denominator in both molarity and molality practically the same, leading to their values being almost identical.
Let’s take a real-world example. Imagine you add a tiny pinch of salt to a large glass of water. The salt dissolves, but it hardly changes the overall volume of the water. In this case, the molarity and molality of the salt solution would be very close.
Remember, the approximation of molarity and molality being equal only holds true for dilute aqueous solutions. As the solution becomes more concentrated, the solute’s contribution to the volume becomes more significant, and the difference between molarity and molality becomes more pronounced.
How are molarity and molality the same?
Let’s think of it like this: Imagine you have a glass of water. If you add sugar to the water, you are changing the molarity of the solution. But if you add ice to the glass, the molality of the solution will not change because the mass of the solvent (water) remains the same.
Another way to understand the difference is to think about how molarity and molality are calculated. Molarity is calculated by dividing the moles of solute by the volume of the solution in liters. Molality is calculated by dividing the moles of solute by the mass of the solvent in kilograms.
When you are working with solutions, it’s important to know the difference between molarity and molality. You can use molarity to determine the concentration of a solution, but you can’t use it to determine the freezing point or boiling point of a solution. To determine these properties, you need to use molality.
In which case molarity is equal to molality?
Let’s break this down:
Molarity is the number of moles of solute per liter of solution (mol/L).
Molality is the number of moles of solute per kilogram of solvent (mol/kg).
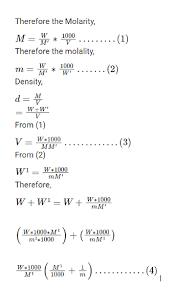
In very dilute solutions, the mass of the solute is negligible compared to the mass of the solvent (water). Therefore, the mass of the solution is practically the same as the mass of the solvent. Since the density of water is close to 1 kg/L, the volume of the solution is approximately equal to the mass of the solvent. As a result, the denominator in the molarity and molality equations becomes practically the same, leading to their values being nearly equal.
However, it’s important to note that this approximation holds true only for very dilute solutions. As the concentration of the solute increases, the difference between molarity and molality becomes more significant. This is because the volume of the solution deviates from the volume of the solvent, and the mass of the solute becomes more considerable.
Why is molarity and molality the same if the solvent is water?
Molarity measures the concentration of a solution by considering the number of moles of solute per liter of solution. Molality, on the other hand, measures the concentration of a solution by considering the number of moles of solute per kilogram of solvent.
The key to understanding why they’re nearly identical in water is density. Water has a density of approximately 1 kg/L at room temperature. This means that 1 liter of water weighs about 1 kilogram. Since molarity is based on liters and molality on kilograms, the “per L” in molarity essentially becomes equal to the “per kg” in molality when the solvent is water.
Now, let’s think about other solvents. Take ethanol, for example. Ethanol has a density of about 0.789 kg/L. This means that 1 liter of ethanol weighs 0.789 kilograms. In this case, a 1 M solution of something dissolved in ethanol would be 0.789 m because the volume (1 L) and mass (0.789 kg) of the solvent are not equal.
In summary, the close relationship between molarity and molality in water is a direct consequence of its convenient density of 1 kg/L. This makes it much easier to work with these concentration units when dealing with aqueous solutions. For other solvents, the density will come into play, leading to a difference between molarity and molality.
In which condition molarity and molality of a solution are nearly the same?
Molarity is defined as the number of moles of solute per liter of solution. This means it considers the total volume of the solution, including both the solute and the solvent.
Molality, on the other hand, is defined as the number of moles of solute per kilogram of solvent. It only considers the mass of the solvent.
In dilute solutions, the volume of the solute is negligible compared to the volume of the solvent. This is especially true when the solvent is water, as it has a relatively high density. Since the volume of the solution is almost entirely due to the water, the difference between the volume of the solution and the mass of the water becomes insignificant. Consequently, molarity and molality are nearly the same in these cases.
Here’s an example: imagine you have a solution of 1 gram of sugar (solute) dissolved in 1000 grams of water (solvent). In this scenario:
Molarity: You’d need to calculate the volume of the entire solution (sugar + water) to determine molarity.
Molality: You’d only need to consider the mass of the water (1000 grams), making the calculation simpler.
Because the sugar’s volume is small compared to the water’s volume, the calculated values for molarity and molality will be very similar.
It’s crucial to remember that this approximation only holds true for dilute solutions with water as the solvent. As the concentration of the solute increases, or if a solvent other than water is used, the difference between molarity and molality becomes more significant. This is because the volume of the solute becomes more substantial in concentrated solutions, and the density of the solvent can vary greatly.
Under what conditions are the molarity and molality of a solution nearly the same and does a change in temperature influence their values?
Molality is the number of moles of solute per kilogram of solvent. Molarity, on the other hand, is the number of moles of solute per liter of solution.
The key difference lies in their dependence on temperature. Molality remains constant regardless of temperature changes because it’s based on mass, which doesn’t change with temperature. Molarity, however, is based on volume, which expands when heated and contracts when cooled. This means that molarity changes with temperature fluctuations.
Now, let’s explore when molarity and molality are nearly the same. This happens when the density of the solution is close to 1 kg/L. Think of water – its density is almost exactly 1 kg/L at 4°C. In such cases, the volume of the solution is roughly equal to the mass of the solvent, making molarity and molality practically the same.
Let me illustrate this with an example: imagine a solution where the solvent is water. If the solution is very dilute, meaning it contains a small amount of solute, the density of the solution will be very close to the density of water. In this scenario, the mass of the solvent will be nearly equal to the volume of the solution. As a result, molality and molarity will be quite similar.
However, it’s important to remember that this close approximation only holds true for dilute solutions with a solvent density close to 1 kg/L. As the concentration of the solute increases or the solvent’s density deviates from 1 kg/L, the difference between molarity and molality becomes more pronounced.
To summarize, molality is a more reliable measure of concentration when dealing with temperature variations, as it remains constant. Molarity, while convenient for many applications, is sensitive to temperature changes. In dilute solutions with a solvent density close to 1 kg/L, molarity and molality can be considered nearly equivalent. However, for more concentrated solutions or those with solvents that have significantly different densities, the difference between these two concentration units becomes more significant.
What is the relation between molarity and molality with temperature?
Molarity is defined as the number of moles of solute per liter of solution. As temperature increases, the volume of the solution expands due to the increased kinetic energy of the molecules. Since molarity is based on the volume of the solution, this expansion leads to a decrease in molarity. Think of it this way: the same amount of solute is now spread out in a larger volume, leading to a lower concentration.
Molality, on the other hand, is defined as the number of moles of solute per kilogram of solvent. Since molality is based on the mass of the solvent, which is not affected by temperature changes, molality remains constant even as temperature changes. The mass of the solvent doesn’t change with temperature, so the concentration in terms of molality remains the same.
To summarize, molarity decreases with increasing temperature because the volume of the solution expands. Molality, however, remains constant with temperature changes because it is based on the mass of the solvent, which is not affected by temperature.
See more here: How Are Molarity And Molality The Same? | Under What Conditions Are Molarity And Molality Approximately The Same
What is the difference between molality and molarity?
Molality (m) is defined as the number of moles of solute per kilogram of solvent. So, if you have a solution with 1 mole of solute dissolved in 1 kilogram of solvent, the molality of the solution is 1 m.
Molarity, on the other hand, is defined as the number of moles of solute per liter of solution. So, if you have a solution with 1 mole of solute dissolved in 1 liter of solution, the molarity of the solution is 1 M.
The key difference lies in the denominator: molality uses the mass of the solvent, while molarity uses the volume of the solution.
This difference becomes important when you consider that the volume of a solution can change with temperature, while the mass of the solvent remains constant. For this reason, molality is often preferred for solutions where temperature changes are expected or when precise measurements of concentration are required.
Let’s consider an example. Imagine you have a solution of sugar in water. If you heat this solution, the volume of the solution will expand, leading to a decrease in the molarity. However, the mass of the water (the solvent) remains the same, so the molality of the solution remains unchanged.
In the case of dilute aqueous solutions, the difference between molality and molarity is often negligible. This is because the mass of the solvent is almost equal to the mass of the solution, and the volume of the solution is very similar to the volume of the solvent. As a result, the two values are often considered interchangeable in these specific cases.
What is molality in chemistry?
Molality is a way to measure the concentration of a solution. It tells us how many moles of solute are dissolved in one kilogram of solvent.
Why is this important? Well, unlike molarity (which uses the volume of the solution), molality stays consistent even when the temperature changes. This is because the volume of a solution can be affected by temperature, but the mass of the solvent remains the same.
For example, imagine you have a sugar solution. If you heat it up, the volume of the solution might increase a bit. But the amount of sugar (the solute) and the mass of water (the solvent) won’t change. This means the molality of the solution stays the same, even though the molarity might change.
Let’s break down the definition:
Solute: The substance that gets dissolved (like sugar in our example).
Solvent: The substance that does the dissolving (like water).
Solution: The mixture formed when the solute dissolves in the solvent.
How do we write molality?
We use a lowercase “m” to denote molality. For example, a 1.0 m solution would have 1 mole of solute per kilogram of solvent.
Let’s think about it in terms of our sugar solution:
* If you dissolve 1 mole of sugar (about 342 grams) in 1 kilogram (1000 grams) of water, you have a 1.0 m sugar solution.
Here’s why molality is helpful:
* It helps us understand the relative amounts of solute and solvent in a solution.
* It is useful for studying physical properties of solutions that are dependent on the concentration of the solute, such as freezing point depression and boiling point elevation.
Remember: Molality is a great tool for describing the concentration of a solution and for understanding how temperature changes affect it. It’s a crucial concept in chemistry, especially when studying properties that depend on the concentration of the solute.
When is molarity used?
Molarity (M) tells us the number of moles of solute per liter of solution. It’s a pretty common way to express concentration, especially in chemistry and biology. Molarity is super useful when you’re working with solutions at a specific temperature because it takes into account the volume of the entire solution, not just the solvent.
Think of it this way: Imagine you’re making a pitcher of lemonade. Molarity would tell you how many moles of sugar are in each liter of lemonade.
Now, let’s talk about why molarity is so handy:
Easy calculations:Molarity simplifies calculations when you need to determine the amount of a substance you need for a reaction. You can use molarity to calculate the moles of solute or the volume of solution required for your experiment.
Consistent results: When working with solutions at a specific temperature, molarity helps ensure you’re using consistent amounts of the dissolved substance in your experiments.
Let’s go back to our lemonade example: If you use molarity to measure the sugar concentration, you know that no matter how much lemonade you make, each liter will have the same amount of sugar. This consistency is crucial for getting accurate results.
Molarity is a really useful tool for chemists and scientists, and now you know how it works and why it’s so important!
Why are molality and molarity closest to each other?
Molality is defined as the number of moles of solute per kilogram of solvent, while molarity is defined as the number of moles of solute per liter of solution. When the solute concentration is low, the solvent makes up most of the solution’s volume. In this scenario, the mass of the solvent is very close to the volume of the solution (since the density of water is nearly 1 g/mL). Therefore, molality and molarity will be very similar.
Think of it this way: imagine adding a tiny amount of sugar to a large glass of water. The sugar (our solute) is so small compared to the water (our solvent) that it hardly affects the overall volume of the mixture. The mass of the water is practically the same as the volume of the solution. This is why the values for molality and molarity will be similar in this dilute solution.
However, as the amount of solute increases, the difference between molality and molarity becomes more pronounced. This is because the solute now contributes significantly to the overall volume of the solution. The volume of the solution is greater than the mass of the solvent due to the volume occupied by the solute. Consequently, the values of molality and molarity start to diverge.
For example, if we add a lot of sugar to the water, the sugar will take up a considerable amount of space. The solution’s volume will be larger than the water’s mass, leading to differences in the values of molality and molarity.
In conclusion, when the solute concentration is low, molality and molarity are nearly equal because the solute’s contribution to the solution’s volume is negligible. As the solute concentration increases, the difference between molality and molarity becomes more apparent because the solute starts to significantly influence the solution’s volume.
See more new information: linksofstrathaven.com
Under What Conditions Are Molarity And Molality Approximately The Same?
Hey there, chemistry enthusiasts! Today we’re diving into the fascinating world of concentration units, specifically molarity and molality.
While these two terms might sound similar, they actually represent different ways of expressing the concentration of a solution.
Molarity (M) is defined as the number of moles of solute per liter of solution.
Molality (m) is defined as the number of moles of solute per kilogram of solvent.
Now, you might be wondering, when can we consider these two measures to be approximately the same?
Well, the key lies in the density of the solution.
Let’s break it down:
When the density of the solution is close to 1 kg/L (or 1 g/mL), molarity and molality will be approximately equal.
Think about it:
* If the density is close to 1 kg/L, 1 liter of solution will have a mass close to 1 kilogram.
* Since the solvent makes up the majority of the solution, the mass of the solvent will be very close to the mass of the solution.
* This means that the denominator in both molarity and molality calculations will be practically the same.
For example, if you’re dealing with a solution made up of water as the solvent, the density of water is close to 1 g/mL. In such cases, you can safely use molarity and molality interchangeably, as the difference between the two will be negligible.
However, when the density of the solution deviates significantly from 1 kg/L, the difference between molarity and molality becomes more pronounced.
Here’s an example:
Let’s say you have a concentrated solution of sugar dissolved in water. The density of this solution will be higher than 1 kg/L because the presence of the dissolved sugar increases the overall mass of the solution. In this case, the molality will be higher than the molarity because the denominator in the molality calculation (mass of solvent) will be smaller than the denominator in the molarity calculation (volume of solution).
So, to summarize:
Molarity and molality are approximately the same when the density of the solution is close to 1 kg/L.
This is usually the case for dilute solutions, especially those with water as the solvent.
When the density of the solution deviates significantly from 1 kg/L, the difference between molarity and molality becomes more pronounced.
In general, it’s best to use molality when working with solutions that have a high concentration of solute or when the density of the solution is significantly different from 1 kg/L.
Molarity is more convenient to use in everyday laboratory settings because volumes are easier to measure than masses.
Let’s look at some real-world examples where this concept comes into play:
Preparing solutions for biological experiments: Often, researchers need to prepare solutions with precise concentrations. When working with dilute solutions of biological molecules in water, the density of the solution will be very close to 1 kg/L. Therefore, they can safely use either molarity or molality to express the concentration.
Calculating the concentration of electrolytes in blood: Blood is a complex mixture containing various electrolytes. The density of blood is slightly higher than 1 kg/L. However, the difference in concentration expressed in terms of molarity and molality will be very small in this case.
Manufacturing industrial chemicals: In industrial processes where concentrated solutions are used, the density of the solutions can deviate significantly from 1 kg/L. In such scenarios, molality is preferred over molarity to ensure accurate concentration measurements.
To make the concept clearer, let’s consider a few FAQs:
FAQs
Q: Why is molality preferred for solutions with high concentrations of solute?
A: Molality is preferred for solutions with high concentrations of solute because it is independent of temperature. The volume of a solution can change with temperature, but the mass of the solvent remains constant. Molality is based on the mass of the solvent, making it a more accurate measure of concentration in situations where temperature changes are significant.
Q: What are some practical applications of molality in chemistry?
A: Molality is commonly used in:
Colligative property calculations: Molality is used to determine properties of solutions such as freezing point depression, boiling point elevation, and osmotic pressure.
Electrolyte concentration measurements: In electrochemistry, molality is used to express the concentration of electrolytes in solutions.
Thermodynamic calculations: Molality is used in thermodynamic calculations to account for the effect of temperature on solution properties.
Q: What are the advantages and disadvantages of using molarity and molality?
A:
Molarity:
Advantages:
* Easy to measure: Volumes are easier to measure than masses.
* Commonly used in laboratory settings: Molarity is widely used in chemistry labs due to its ease of use.
Disadvantages:
* Temperature-dependent: The volume of a solution can change with temperature, affecting the molarity.
* Not suitable for high-concentration solutions: For highly concentrated solutions, the difference between molarity and molality can be significant.
Molality:
Advantages:
* Temperature-independent: Molality is independent of temperature, making it more accurate for solutions with significant temperature changes.
* Suitable for high-concentration solutions: Molality is the preferred concentration unit for high-concentration solutions.
Disadvantages:
* Requires mass measurements: Determining the mass of the solvent can be more complex than measuring volume.
* Less commonly used in laboratory settings: Molality is less frequently used in laboratory settings compared to molarity.
In conclusion, while both molarity and molality are measures of concentration, they are not always interchangeable. Understanding the conditions under which these measures are approximately the same is crucial for accurate chemical calculations and reliable experimental results.
Remember, when the density of the solution is close to 1 kg/L, molarity and molality will be practically equal. However, when the density deviates from this value, it’s important to choose the appropriate concentration unit to ensure accuracy in your work.
What is the Difference Between Molarity and Molality? – ThoughtCo
Molarity is the ratio of moles to volume of the solution (mol/L) while molality is the ratio of moles to the mass of the solvent (mol/kg). Most of the time, it doesn’t matter which unit of concentration you use. ThoughtCo
Why Is Molality Used Instead of Molarity? – ThoughtCo
Molality is the number of moles of solute per kilogram of solvent. Molarity is the number of moles of solute per liter of solution. If the solvent is water and the concentration of solute ThoughtCo
The Difference Between Molality and Molarity
Both m and M are units of the concentration of a chemical solution. The lowercase m indicates molality, which is calculated using ThoughtCo
5.1 Expressing Solution Concentration – Chemistry LibreTexts
Molality, therefore, has the same numerator as molarity (the number of moles of solute) but a different denominator (kilogram of solvent rather than liter of Chemistry LibreTexts
Difference Between Molarity and Molality – Understand Chemistry …
Molarity is the most common concentration measurement and denoted by the capital letter M. Molarity is the number of moles of something per volume of mixture Science Notes and Projects
Molarity vs. molality (video) | Khan Academy
Learn how molarity and molality differ! The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of Khan Academy
16.11: Molality – Chemistry LibreTexts
Molality differs from molarity only in the denominator. While molarity is based on the liters of solution, molality is based on the kilograms of solvent. Concentrations expressed in Chemistry LibreTexts
Molarity vs Molality: Formula and Definitions
The molality describes the moles of a solute in relation to the mass of a solvent, while the molarity is concerned with the moles of a solute in relation to the volume of a solution. Read on to learn more Technology Networks
3.1: Units of Concentration – Chemistry LibreTexts
Under what conditions are molality and molarity likely to be equal? Is the difference between the two greater when water is the solvent or when the solvent is not water? Chemistry LibreTexts
Molarity, Molality, Volume \U0026 Mass Percent, Mole Fraction \U0026 Density – Solution Concentration Problems
Difference Between Molarity And Molality
Assertion : Molarity \U0026 Molality For Very Dilute Aqueous Solution Is Approximately Same Reason : …
Molarity Vs. Molality | Lab Values And Concentrations | Health \U0026 Medicine | Khan Academy
Under What Condition Molality Is Equal To Or Greater Than Or Less Than Molarity?
When Are Molality \U0026 Molarity Equally Dilute? : Chem Class
Molarity Calculation Formula And Example | How To Solve Molarity Problems?
Link to this article: under what conditions are molarity and molality approximately the same.
See more articles in the same category here: https://linksofstrathaven.com/how
