Is alkyl benzoate a carcinogen?
The good news is that these ingredients are generally considered safe. Extensive testing has shown they’re not harmful to reproduction or development, and they don’t cause genetic damage in most tests. Importantly, they haven’t been linked to cancer. This means that alkyl benzoates are safe when used as intended at the concentrations typically found in products.
But let’s break down what this means:
Not Reproductive or Developmental Toxicants: This means they don’t harm a developing fetus or a person’s reproductive system. Think of it like a “green light” for safe use during pregnancy or for those trying to conceive.
Not Genotoxic in Most Assays: Genotoxicity is the ability to damage DNA. Our DNA is the blueprint for our bodies. When DNA is damaged, it can lead to diseases like cancer. So, the fact that alkyl benzoates are not genotoxic in most tests means they are unlikely to cause DNA damage and therefore unlikely to lead to cancer.
Not Carcinogenic: This is the big one – they don’t cause cancer. This is reassuring because cancer is a serious concern.
How can we be so confident?
It’s not just about one study. Scientists use a combination of testing methods, looking at different aspects of safety. They consider how the ingredients interact with our bodies, how they break down, and how they might affect our cells. This process helps them determine whether something is safe for long-term use.
Important Note: While alkyl benzoates are generally considered safe, it’s always a good idea to be mindful of what you’re using, especially if you have sensitive skin or allergies. If you’re concerned about a particular product, read the label carefully and consider consulting a dermatologist or other healthcare professional.
Is alkyl benzoate safe?
Let’s talk about alkyl benzoate. You’re likely wondering if it’s safe, and that’s a smart question!
According to the Material Safety Data Sheet (MSDS), alkyl benzoate is considered safe for use in cosmetics and personal care products. It’s a common ingredient in things like lotions, soaps, and perfumes. However, like many ingredients, it can cause eye irritation.
So, alkyl benzoate is generally safe, but it’s always a good idea to be cautious. If you have sensitive skin, you might want to test a product with alkyl benzoate on a small area of your skin first. If you experience any irritation, stop using the product and talk to your doctor.
Alkyl benzoates are a group of esters derived from benzoic acid. They have a wide range of uses, including as fragrances, plasticizers, and solvents. In cosmetics and personal care products, alkyl benzoates are often used as emollients (to soften and smooth skin) and solvents (to help other ingredients blend).
While alkyl benzoates are generally safe for use in cosmetics and personal care products, there are a few things to keep in mind:
Eye irritation: As mentioned above, alkyl benzoates can cause eye irritation. It’s important to avoid getting them in your eyes. If you do get them in your eyes, flush them with water for at least 15 minutes.
Skin sensitivity: Some people may be sensitive to alkyl benzoates, and may experience skin reactions such as redness, itching, or dryness. If you have sensitive skin, it’s a good idea to test a product with alkyl benzoates on a small area of your skin first.
Allergic reactions: While rare, some people may have an allergic reaction to alkyl benzoates. If you experience any symptoms of an allergic reaction, such as hives, swelling, or difficulty breathing, seek medical attention immediately.
Overall, alkyl benzoates are a safe and effective ingredient when used appropriately. However, it’s important to be aware of the potential side effects and to use products with alkyl benzoates with caution.
What is alkyl benzoate in sunscreen?
First, C12-15 Alkyl Benzoate is very soluble. That means it mixes well with other ingredients. This is important because it helps keep the sunscreen’s active ingredients spread evenly throughout the product. This makes sure the sunscreen works properly and protects your skin effectively.
Not only that, studies show that C12-15 Alkyl Benzoate can even help make certain sunscreen actives more powerful. This is great news for our skin!
So, what does that mean for you? It means that C12-15 Alkyl Benzoate plays an important role in making sunscreens effective and easy to use.
C12-15 Alkyl Benzoate is a type of ester that’s made from a combination of fatty acids and benzoic acid. It’s a clear, oily liquid that’s not easily soluble in water, but mixes very well with oils.
This makes it an ideal choice for use in sunscreens, as it helps to create a smooth and even consistency. It also helps to keep the sunscreen from separating or becoming clumpy over time.
The solubility of C12-15 Alkyl Benzoate is crucial for sunscreen formulations. It allows the sunscreen’s active ingredients to disperse evenly, ensuring that the product works effectively. This is especially important for chemical sunscreens, which need to absorb into the skin to work properly.
C12-15 Alkyl Benzoate also helps to improve the texture and feel of sunscreens. It can help to make the sunscreen more spreadable and less sticky. This makes it more enjoyable to apply and allows for a more even application.
In addition to its role as a solvent, C12-15 Alkyl Benzoate can also act as a skin conditioning agent. This means that it can help to soften and moisturize the skin. This is a welcome bonus for many sunscreen users, as it can help to prevent dryness and irritation.
C12-15 Alkyl Benzoate is generally considered safe for use in sunscreens. However, as with any ingredient, some individuals may experience allergic reactions. If you have sensitive skin, it’s always a good idea to test a small amount of the product on a patch of skin before using it all over.
Is benzyl the same as benzoate?
Benzyl alcohol and benzoic acid are related compounds, but they have different functional groups. Benzyl alcohol has a hydroxyl group (-OH), while benzoic acid has a carboxyl group (-COOH). Now, sodium benzoate, calcium benzoate, and potassium benzoate are all salts of benzoic acid.
What about benzyl benzoate? Well, benzyl benzoate is an ester. It’s formed when benzyl alcohol and benzoic acid react.
Think of it this way:
Benzyl alcohol is like a simple building block.
Benzoic acid is a slightly more complex building block.
Sodium benzoate, calcium benzoate, and potassium benzoate are like modified versions of benzoic acid.
Benzyl benzoate is like a combination of benzyl alcohol and benzoic acid, joined together in a special way.
Let’s put it into simpler terms. You can imagine a Lego® set. Benzyl alcohol is like a basic brick, while benzoic acid is like a brick with a special connector. Sodium benzoate, calcium benzoate, and potassium benzoate would be like different versions of the brick with the connector. Benzyl benzoate would be like two bricks connected together using the connector.
Here’s the key point: Benzyl refers to a specific group of atoms (the benzyl group, which is a benzene ring attached to a -CH2- group). Benzoate refers to a group of atoms derived from benzoic acid.
The two are related because they both have a benzene ring, but they have different functional groups and different properties.
Is benzene and benzoate the same thing?
While benzoate and benzene are not the same, there is a potential for benzene to form in some foods that contain sodium benzoate and ascorbic acid (vitamin C). This reaction is most likely to occur in acidic foods and beverages, such as diet sodas. The amount of benzene that is formed is typically very low, but it’s still important to be aware of this potential reaction.
Here’s what you need to know about the formation of benzene from sodium benzoate and ascorbic acid:
How it happens: Benzene forms when sodium benzoate reacts with ascorbic acid in the presence of heat and acidic conditions.
Where it happens: This reaction is most likely to occur in foods and beverages that are acidic, such as diet sodas, fruit juices, and some salad dressings.
How much benzene forms: The amount of benzene that forms is typically very low and is unlikely to pose a health risk. However, the FDA has set a limit for benzene in food and beverages, and companies are required to monitor their products for benzene levels.
What you can do: If you are concerned about the potential formation of benzene in foods, you can choose products that do not contain sodium benzoate or ascorbic acid. You can also choose products that are not acidic or that are not exposed to high temperatures.
It’s important to note that the formation of benzene from sodium benzoate and ascorbic acid is a complex issue and there is still much that we don’t know. However, the FDA and other regulatory agencies are actively monitoring the situation and working to ensure that food and beverages are safe for consumers.
Is benzoate cancerous?
Benzene has been linked to cancer in some studies, but it’s important to note that these studies were conducted under specific conditions. The amount of benzene formed from the combination of sodium benzoate and vitamin C in food is typically very small, and it’s unlikely to pose a significant health risk.
Here’s a breakdown of what’s important to understand:
Sodium Benzoate: This is a common food preservative found in many products, including sodas, sauces, and fruit juices. It helps prevent spoilage and extend shelf life.
Ascorbic Acid (Vitamin C): This is a natural vitamin that’s essential for our health. It’s often added to foods as a supplement.
Benzene Formation: When sodium benzoate and ascorbic acid are combined in the presence of heat or certain acidic conditions, a small amount of benzene can form.
Benzene and Cancer: While benzene has been linked to cancer in some studies, it’s important to remember that these studies were conducted using high doses of benzene over long periods. The amount of benzene formed in food is generally very low, and it’s unlikely to pose a significant health risk.
Regulation: Food safety agencies like the FDA (Food and Drug Administration) closely monitor the levels of benzene in food and beverages. They have set limits to ensure that the amount of benzene in food is below a safe level.
It’s important to keep in mind that the formation of benzene from sodium benzoate and vitamin C is a complex process. While it’s something to be aware of, the risk to consumers is generally considered low.
If you’re concerned about benzene levels in your food, you can:
Read food labels: Look for products that don’t contain sodium benzoate or ascorbic acid.
Choose fresh foods: Fresh fruits and vegetables don’t contain preservatives like sodium benzoate.
Limit processed foods: Processed foods are more likely to contain preservatives like sodium benzoate.
By making these simple changes, you can help reduce your exposure to benzene and other potentially harmful chemicals.
What are the benefits of alkyl benzoate?
Alkyl benzoate is a synthetic ingredient, but don’t let that scare you! It’s actually very safe and well-tolerated by most people. It’s made from esters, which are naturally occurring compounds found in many fruits and plants. This makes it a great choice for those with sensitive skin.
The best part? It leaves a silky, non-oily finish. This means you’ll enjoy the benefits of a deeply moisturized complexion without feeling greasy or heavy. This makes it perfect for a wide range of products, from lotions and creams to foundations and eyeshadows.
Alkyl benzoate is a truly unique ingredient. Because it’s both soluble in oil and oil-like ingredients, it’s able to create a balanced and luxurious feel for products. It creates a smooth, silky, and non-oily finish that will leave your skin feeling soft and supple. So next time you’re looking for a product that’s both effective and gentle, look for C12-15 alkyl benzoate in the ingredient list. You won’t be disappointed!
See more here: Is Alkyl Benzoate Safe? | Is Alkyl Benzoate The Same As Benzene
Are alkyl benzoates toxic?
Isononyl benzoate has been studied, and we can look at those findings. We also have data on benzoic acid, sodium benzoate, and the alcohol metabolites of alkyl benzoates. Let’s dive into what we know about these related compounds.
Isononyl benzoate, a common ingredient in cosmetics and personal care products, has been studied for its potential effects on reproduction and development. One study found that isononyl benzoate did not cause reproductive toxicity in rats. However, it’s important to note that this study was conducted at relatively low doses, and further research is needed to understand its potential effects at higher doses or during different stages of development.
Benzoic acid, a naturally occurring compound found in many fruits and vegetables, is often used as a preservative in food and cosmetics. Studies have shown that benzoic acid is generally considered safe for human use. However, some individuals may experience allergic reactions or skin irritation when exposed to benzoic acid.
Sodium benzoate, the sodium salt of benzoic acid, is another common preservative used in food and cosmetics. Like benzoic acid, sodium benzoate is generally considered safe for human use. However, some individuals may experience allergic reactions or other adverse effects when exposed to sodium benzoate.
The alcohol metabolites of alkyl benzoates are the breakdown products of these compounds in the body. These metabolites are generally considered to be safe, but further research is needed to fully understand their potential effects.
It’s important to note that these findings are based on limited studies, and more research is needed to fully understand the potential reproductive and developmental toxicity of alkyl benzoates. It’s always a good idea to talk to your doctor or a healthcare professional if you have any concerns about the safety of any ingredient in your cosmetics or other products.
Are alkyl benzoate esters safe?
The good news is, alkyl benzoate esters have been thoroughly studied, and in the concentrations used in cosmetics, they’re considered safe. They play a variety of roles in your favorite beauty products:
Fragrance ingredients: They add pleasant scents.
Skin-conditioning agents – emollients: They help to soften and moisturize skin.
Skin-conditioning agents – miscellaneous: They can help improve the feel and texture of your skin.
Preservatives: They help extend the shelf life of your products by preventing microbial growth.
Solvents: They help dissolve other ingredients in the product.
Plasticizers: They help make the product more flexible and spreadable.
Alkyl benzoate esters are derived from natural sources, like plants and fruits. They’re generally well-tolerated by most people, but as with any ingredient, some individuals might experience sensitivity or irritation.
Here’s a deeper dive into some specific examples:
Benzyl benzoate: This ester is often used in sunscreens and insect repellents. It’s a common ingredient in perfumes as well.
Butyl benzoate: This is a versatile ester used in a wide range of cosmetics, from lotions and creams to hair care products.
Ethyl benzoate: This ester is known for its pleasant, sweet scent. It’s often used in fragrances and perfumes.
If you’re concerned about any particular ingredient, it’s always best to check the product label and patch test a small area of skin before applying it to your entire body. This helps you identify any potential sensitivities.
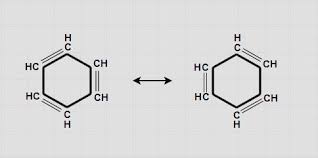
Why is benzene called alkyl-substituted benzene?
Let’s break down why benzene is called alkyl-substituted benzene.
Benzene: Benzene is a fundamental aromatic hydrocarbon with a ring structure of six carbon atoms and six hydrogen atoms. It’s often depicted as a hexagon with a circle inside, representing the delocalized electrons.
Alkyl Group: An alkyl group is a branched or unbranched chain of carbon and hydrogen atoms derived from an alkane by removing one hydrogen atom. Think of it as a building block that can be attached to other molecules. Common alkyl groups include methyl (CH3), ethyl (CH2CH3), propyl (CH2CH2CH3), and so on.
Alkyl-Substituted Benzene: When an alkyl group attaches to the benzene ring, it’s called alkyl-substituted benzene. The benzene ring acts as the base structure, and the alkyl group becomes the substituent. Think of it like decorating a cake – benzene is the cake, and the alkyl group is the frosting!
Let’s look at some examples:
Toluene: This is methyl-substituted benzene. The benzene ring has a methyl group (CH3) attached to it.
Ethylbenzene: This is ethyl-substituted benzene, with an ethyl group (CH2CH3) attached to the benzene ring.
Isopropylbenzene (Cumene): This is isopropyl-substituted benzene, with an isopropyl group (CH(CH3)2) attached to the benzene ring.
The size of the alkyl group matters in naming. If the alkyl group is smaller than the benzene ring (six or fewer carbons), we typically name it as an alkyl-substituted benzene. This naming system helps chemists quickly identify and understand the structure of these organic molecules.
Which alkyl benzoates have comedogenicity?
It’s true that some alkyl benzoates have been studied for their potential to cause comedogenicity. Here’s what we know:
Lauryl alcohol (50% in mineral oil) and myristyl alcohol (50% in mineral oil) had slight comedogenicity. This means they might slightly clog pores.
Octyl alcohol (100%) had strong comedogenicity, which means it’s more likely to clog pores.
It’s important to note that the research on alkyl benzoates and their impact on comedogenicity is still ongoing. More studies are needed to fully understand how these ingredients affect different skin types.
Understanding Comedogenicity
Comedogenicity refers to the tendency of a substance to clog pores and lead to breakouts. It’s a complex issue, as different people react differently to various ingredients. What might clog one person’s pores might not affect another person’s skin at all.
Factors Affecting Comedogenicity
Several factors can influence how likely a substance is to cause comedogenicity, including:
The chemical structure of the ingredient: Some chemical structures are more likely to clog pores than others.
The concentration of the ingredient in a product: Higher concentrations of an ingredient can increase the risk of comedogenicity.
Individual skin type: People with oily or acne-prone skin are more susceptible to breakouts from ingredients that have comedogenic potential.
What to Look for in Products
If you’re concerned about comedogenicity, it’s always best to look for products formulated with ingredients that are known to be non-comedogenic or low-comedogenic. Many skincare brands label their products to indicate comedogenicity.
It’s also a good idea to patch test new products on a small area of your skin before using them on your entire face. This allows you to see if you have any adverse reactions.
Remember, everyone’s skin is different. If you’re experiencing breakouts, it’s essential to consult with a dermatologist to get personalized advice on how to manage your skin concerns.
See more new information: linksofstrathaven.com
Is Alkyl Benzoate The Same As Benzene?
Okay, so you’re wondering if alkyl benzoate is the same thing as benzene. Let’s dive into this.
The short answer is no, they’re not the same.
Alkyl benzoates and benzene are two distinct chemical compounds with different structures, properties, and uses.
Benzene is a simple aromatic hydrocarbon with the molecular formula C6H6. It’s a colorless, flammable liquid with a sweet smell. You might find it used in the production of plastics, resins, and other chemicals.
Alkyl benzoates, on the other hand, are a class of organic compounds that contain a benzene ring (that’s the C6H6 part) with an alkyl group attached to a carboxyl group (-COOH). This structure looks like this:
[Image: Basic alkyl benzoate structure, with the benzene ring, alkyl group, and carboxyl group clearly labelled.]Alkyl benzoates are esters formed by the reaction of benzoic acid with an alcohol. They’re generally pleasant-smelling liquids that are commonly used in perfumes, cosmetics, and as flavorings.
Here’s a breakdown of the key differences:
| Feature | Benzene | Alkyl Benzoate |
|—————–|———————————————–|—————————————————|
| Structure | Simple aromatic ring (C6H6) | Benzene ring with an alkyl group and carboxyl group |
| Properties | Colorless, flammable, sweet smell | Generally pleasant-smelling liquids |
| Uses | Plastics, resins, chemicals | Perfumes, cosmetics, flavorings |
| Toxicity | Classified as a human carcinogen | Generally considered safe for use in small amounts |
So, while both benzene and alkyl benzoates contain a benzene ring, they’re not the same thing. Benzene is a basic hydrocarbon, while alkyl benzoates are complex esters with specific properties and uses.
Why the Confusion?
You might be asking yourself, “If they’re not the same, why is there even a connection?” Well, it comes down to the benzene ring. Both benzene and alkyl benzoates contain this aromatic ring, which is a key structural feature that gives these molecules their unique properties. This common structural feature is why you might see them mentioned together or grouped under the umbrella term “aromatic compounds”.
Important Note:
It’s important to remember that benzene is a known human carcinogen. This means that prolonged exposure to benzene can increase the risk of developing cancer. It’s crucial to handle benzene carefully and take necessary safety precautions. Alkyl benzoates, however, are generally considered safe for use in small amounts, but as with any chemical, it’s always best to check the specific safety data sheet for the compound you’re working with.
FAQs
1. Is it okay to use alkyl benzoates if I’m sensitive to benzene?
Yes, generally speaking, alkyl benzoates are safe for use, even if you’re sensitive to benzene. This is because the structure and properties of alkyl benzoates are different from benzene. However, it’s always best to check the ingredient list of any product you’re using, especially if you have known sensitivities or allergies.
2. Are there different types of alkyl benzoates?
Yes! You’ll find a variety of alkyl benzoates out there, each with its unique properties and uses. Some common examples include:
Methyl benzoate: Found in perfumes and flavorings, known for its sweet floral scent.
Ethyl benzoate: Used in cosmetics and as a solvent.
Benzyl benzoate: Used in perfumes, cosmetics, and as a skin treatment.
3. Can I use alkyl benzoate as a substitute for benzene in a chemical reaction?
Not really. Benzene and alkyl benzoates have very different chemical properties. While they both contain a benzene ring, the presence of the alkyl and carboxyl groups in alkyl benzoates significantly alters their reactivity. You’d need to consider the specific reaction you’re trying to perform and choose the appropriate reagent based on its properties and compatibility.
4. What are some other common aromatic compounds besides benzene and alkyl benzoates?
Here are a few more:
Toluene: A common solvent, often used in paint thinners and adhesives.
Phenol: Used in the production of plastics, resins, and pharmaceuticals.
Aniline: A precursor to many dyes and pharmaceuticals.
5. Where can I find more information about benzene and alkyl benzoates?
You can find detailed information on these compounds in a variety of sources, including:
Chemical databases: Databases like PubChem and ChemSpider offer detailed information on chemical structures, properties, and uses.
Scientific journals: Publications like the Journal of Organic Chemistry and the Journal of Chemical Education are excellent resources for in-depth research.
Safety data sheets (SDSs): Always consult the SDS for a specific compound to learn about its hazards, safety precautions, and handling procedures.
Understanding the differences between benzene and alkyl benzoates is crucial for making informed decisions about their use and safety. Remember, while they share a common structural feature, they are distinct compounds with different properties and uses.
Benzene vs. Benzoate – What’s the Difference? | This vs. That
Benzene and benzoate are two organic compounds that share a similar chemical structure but differ in their properties and applications. Understanding the attributes of these compounds is crucial in various fields, including chemistry, pharmaceuticals, and thisvsthat.io
Benzene vs. Benzoate — What’s the Difference?
Benzene is a volatile, aromatic hydrocarbon compound. Benzoate is a salt or ester of benzoic acid. Both are related but differ in their chemical structures and uses. Ask Difference
Benzene vs. Benzoate: What’s the Difference?
Key Differences. Benzene is a colorless, volatile liquid hydrocarbon, forming a ring structure, used in the manufacture of numerous chemicals. Benzoate is a Difference Wiki
Safety Assessment of Alkyl Benzoates as Used in Cosmetics
alkyl benzoates. Introduction. Alkyl benzoate esters function in cosmetics as fragrance ingre-dients, skin-conditioning agents—emollient, skin-conditioning SAGE Journals
15.1: Naming the Benzenes – Chemistry LibreTexts
If the alkyl group attached to the benzene contains seven or more carbons the compounds is named as a phenyl substituted alkane. The name phenyl (C 6 H 5 -)is often abbreviated (Ph) and comes from the Chemistry LibreTexts
Is alkyl-benzoate the same as benzene? | Quizlet
Alkyl benzoate and benzene are two different but related chemical compounds. Benzene is an aromatic hydrocarbon. It has one aromatic ring. On the other hand, alkyl-benzoate Quizlet
Safety assessment of alkyl benzoates as used in cosmetics
The functions of alkyl benzoates in cosmetics include fragrance ingredients, skin-conditioning agents–emollient, skin-conditioning agents–miscellaneous, preservatives, PubMed
C12-15 Alkyl Benzoate – Paula’s Choice
C12-15 alkyl benzoate is a skin-softening, moisture-sealing emollient that lends additional benefits for cosmetic formulas. Learn more at Paula’s Choice. Paula’s Choice
Safety Assessment of Alkyl Benzoates as Used in
Alkyl benzoate esters function in cosmetics as fragrance ingredients, skin-conditioning agents—emollient, skin-conditioning agents—miscellaneous, preservatives, solvents, and plasticizers. SAGE Journals
Cake को Microscope में देखने पर 😱😱 | #Shorts
How To Make Benzene
Isomers Explained #Chemistry #Organicchemistry #Shorts
Fake Blood That Is Chemistry Experiment|| Reaction Of Fecl3 With Potassium Thiocyanate Kscn || Short
Making Benzene
Naming Of Benzene Derivatives#Chemistry#Shorts#Benzene#Organicchemistry#Iupacname#Iupac
How To Convert Ethyl Benzene To Benzoic Acid #Shorts
Link to this article: is alkyl benzoate the same as benzene.
See more articles in the same category here: https://linksofstrathaven.com/how
