Do hydrogen bonds occur between hydrogen?
A hydrogen bond (or H-bond) is an electrostatic attraction between a hydrogen atom that’s covalently bonded to a more electronegative atom like oxygen, nitrogen, or fluorine (the donor), and a lone pair of electrons on another electronegative atom (the acceptor).
The hydrogen atom is essentially “pulled” towards the electronegative atom in the donor group, making it slightly positively charged. The electronegative atom in the acceptor group, which has a lone pair of electrons, is slightly negatively charged. This difference in charge creates a weak attraction, which is what we call a hydrogen bond.
So, can hydrogen bonds occur between two hydrogen atoms? The answer is no. Here’s why:
No electronegative atom: Hydrogen bonds require an electronegative atom like oxygen, nitrogen, or fluorine to act as a donor or acceptor. Hydrogen itself is not electronegative enough to participate in this type of bond.
No lone pair of electrons: Hydrogen atoms do not have lone pairs of electrons, which are essential for the formation of hydrogen bonds.
Weak interaction: Hydrogen bonds are relatively weak compared to covalent bonds, so even if two hydrogen atoms were close enough, the attraction between them would not be strong enough to form a bond.
Think of it this way: imagine you have two magnets, but one is much weaker than the other. The stronger magnet can attract the weaker one, but the weaker magnet can’t attract the stronger one. It’s similar with hydrogen bonds. The electronegative atom is like the stronger magnet, and the hydrogen atom is like the weaker magnet. The stronger magnet needs to attract the weaker one to form a hydrogen bond.
So, while hydrogen bonds are crucial for many biological and chemical processes, they don’t form between two hydrogen atoms. It’s a fascinating concept, and understanding how hydrogen bonds work can help you appreciate the intricate nature of chemical interactions.
At which point would hydrogen bonding occur?
Let’s break down this definition to understand it better:
Electronegativity: Electronegativity is a measure of an atom’s ability to attract electrons. Atoms like oxygen (O), nitrogen (N), and fluorine (F) are highly electronegative. When a hydrogen atom is bonded to one of these strongly electronegative atoms, the electrons in the bond are pulled closer to the electronegative atom. This creates a partial positive charge on the hydrogen atom (δ+) and a partial negative charge on the electronegative atom (δ-).
Lone Pair: An electronegative atom like oxygen or nitrogen often has lone pairs of electrons, which are pairs of electrons that are not involved in bonding. These lone pairs can interact with the partially positive hydrogen atom of another molecule.
Hydrogen Bonding: When a hydrogen atom with a partial positive charge (δ+) comes close to an electronegative atom with a lone pair of electrons (δ-), a strong electrostatic attraction forms. This attraction is called a hydrogen bond.
Think of it like this: The hydrogen atom acts like a “bridge” between the two electronegative atoms. It’s attracted to the lone pair on the other atom, forming a strong connection. This is why hydrogen bonds are considered strong intermolecular forces, stronger than typical dipole-dipole interactions.
Examples of Hydrogen Bonding:
Water (H2O): The oxygen atom in water is highly electronegative, creating a partial negative charge on the oxygen and a partial positive charge on the hydrogen atoms. This allows for hydrogen bonds to form between water molecules, giving water its unique properties like high boiling point and surface tension.
DNA: Hydrogen bonds play a crucial role in holding the two strands of DNA together. The nitrogenous bases in DNA form hydrogen bonds with each other, allowing the DNA molecule to maintain its double-helix structure.
Proteins: Hydrogen bonding helps to stabilize the three-dimensional structure of proteins, which is essential for their function.
Key Takeaways:
* Hydrogen bonding is a special type of dipole-dipole attraction.
* It involves a hydrogen atom bonded to a highly electronegative atom interacting with another electronegative atom with a lone pair of electrons.
* It’s a strong intermolecular force that plays a significant role in many biological and chemical processes.
In which situation would hydrogen bonding be present?
Let’s break down why this happens. These electronegative atoms, like oxygen in water (H₂O), have a strong pull on the shared electrons in the bond with hydrogen. This makes the hydrogen atom partially positive (δ+) and the electronegative atom partially negative (δ-). The partially positive hydrogen atom on one molecule is then attracted to the partially negative electronegative atom on another molecule. This attraction is what we call hydrogen bonding.
Hydrogen bonding plays a crucial role in many aspects of chemistry and biology. It’s responsible for the high boiling point of water, the structure of proteins and DNA, and the properties of many organic molecules.
Examples of hydrogen bonding:
Water (H₂O): The hydrogen atoms in water are attracted to the oxygen atom in another water molecule. This is the reason why water has a relatively high boiling point compared to other similar-sized molecules.
DNA: The two strands of DNA are held together by hydrogen bonds between the nitrogenous bases.
Proteins: Hydrogen bonds help to stabilize the three-dimensional structure of proteins.
In essence, hydrogen bonding is a powerful force that plays a key role in the behavior of many molecules.
In what circumstance does a hydrogen bond commonly occur?
You might be wondering, “what makes a hydrogen bond form?” Well, it all comes down to a tug-of-war between atoms for electrons. A hydrogen bond forms when a hydrogen atom is bonded to a highly electronegative atom, like oxygen, nitrogen, or fluorine. These electronegative atoms are like strong magnets, pulling the shared electrons closer to themselves. This leaves the hydrogen atom with a partial positive charge, while the electronegative atom develops a partial negative charge.
Think of it like this: Imagine you have two magnets, one with a north pole and one with a south pole. They are attracted to each other, right? Similarly, the partially positive hydrogen atom is attracted to the partially negative atom on a neighboring molecule. This attraction is what we call a hydrogen bond.
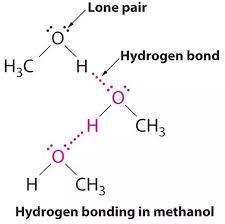
Here’s a simple analogy to help understand how these bonds work: Think of water molecules. Water molecules (H₂O) have two hydrogen atoms and one oxygen atom. The oxygen atom is electronegative, so it pulls the electrons in the covalent bonds closer to itself. This leaves the hydrogen atoms with a partial positive charge and the oxygen atom with a partial negative charge. Because of these charges, water molecules can form hydrogen bonds with each other, forming a strong network of attraction. This is why water is so essential for life; its ability to form hydrogen bonds gives it many unique properties, like a high boiling point and its ability to dissolve many substances.
See more here: At Which Point Would Hydrogen Bonding Occur? | Hydrogen Bonding Occurs Whenever Hydrogen Is Present
What is hydrogen bonding?
It’s important to understand what makes hydrogen bonding so special. You see, hydrogen is the smallest and lightest element, so it packs a powerful punch when it comes to electronegativity. When it bonds with a highly electronegative atom like oxygen, nitrogen, or fluorine, the electron pair is pulled closer to the electronegative atom, creating a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom. This difference in charge is what creates the dipole moment that allows for hydrogen bonding to occur.
Think of it like this: the hydrogen atom is like a little magnet with a positive charge, and the electronegative atom is like another magnet with a negative charge. They attract each other, but it’s a weaker attraction than a full-blown covalent bond. This weak attraction is what makes hydrogen bonding so unique and important.
You might be wondering why it’s called hydrogen bonding when it involves two atoms. Well, that’s because the bond is actually formed between the hydrogen atom of one molecule and the electronegative atom of another molecule. This creates a strong intermolecular force, meaning it’s a force between molecules, rather than within a molecule. This is what gives hydrogen bonding the power to influence many properties of molecules.
Imagine water molecules for a moment. They form hydrogen bonds with each other, which is what makes water so unique. The strong hydrogen bonding in water is responsible for its high boiling point, its ability to dissolve many substances, and its cohesive properties, making it essential for life as we know it.
So, next time you’re sipping on a glass of water, remember the hydrogen bonds that are holding it together. It’s a fascinating example of how these weak interactions can have a big impact on the world around us.
How does a hydrogen bond form?
Now, picture another molecule with an atom that has a slight negative charge. The partially positive hydrogen atom from the first molecule will be attracted to this partially negative atom on the other molecule. This attraction is what we call a hydrogen bond.
So, a hydrogen bond is essentially a special type of dipole-dipole interaction between a partially positive hydrogen atom and a partially negative atom.
Think of it like this: imagine a magnet with a north and south pole. The north pole is attracted to the south pole of another magnet. In a hydrogen bond, the partially positive hydrogen atom acts like the north pole, and the partially negative atom acts like the south pole. These opposite charges attract each other, creating a hydrogen bond.
Hydrogen bonds are quite weak compared to covalent bonds, but they play a critical role in many biological systems. They help stabilize the structures of proteins and DNA, and they are essential for water’s unique properties.
Let’s delve a bit deeper into the concept of electronegativity. Electronegativity is the tendency of an atom to attract electrons towards itself when it’s part of a chemical bond. The higher the electronegativity of an atom, the stronger its pull on electrons. This means that the hydrogen atom in a bond with a highly electronegative atom will have a more significant partial positive charge.
Common examples of highly electronegative atoms include oxygen, nitrogen, and fluorine. They are frequently involved in hydrogen bond formation. For instance, water molecules form hydrogen bonds with each other because the oxygen atom in one water molecule has a partial negative charge and attracts the partially positive hydrogen atoms of other water molecules.
Understanding the concept of electronegativity is crucial for comprehending how hydrogen bonds form. The difference in electronegativity between the hydrogen atom and the other atom in the bond determines the strength of the hydrogen bond.
Which atom is involved in a hydrogen bond?
Most commonly, you’ll find hydrogen bonds forming between hydrogen and oxygen, fluorine, or nitrogen. These three elements are some of the most electronegative elements on the periodic table, making them ideal partners for hydrogen in these special bonds.
You might be wondering how a hydrogen atom can participate in another bond when it’s already part of a covalent bond. This is where the magic of electronegativity comes in. The electronegative atom, like oxygen in water (H₂O), pulls the shared electrons closer to itself, creating a partial negative charge on the oxygen and a partial positive charge on the hydrogen. This uneven distribution of charge makes the hydrogen atom susceptible to being attracted to the lone pairs of electrons on other electronegative atoms nearby, like the oxygen in another water molecule.
This attraction between the partially positive hydrogen of one molecule and the partially negative oxygen of another is what we call a hydrogen bond. This bond is weaker than a covalent bond but still significant, playing a major role in determining the properties of many substances.
For instance, the hydrogen bonds between water molecules are responsible for water’s high boiling point, surface tension, and ability to act as a solvent for many compounds. These properties are essential for life as we know it.
In summary, hydrogen bonds involve a hydrogen atom that is already part of a covalent bond but has a partial positive charge due to the electronegativity of the atom it’s bonded to. This partial positive charge allows the hydrogen atom to form a weak attractive force with a lone pair of electrons on another electronegative atom, like oxygen, fluorine, or nitrogen.
Which atom is more able to form a hydrogen bond?
You know how a hydrogen atom can form a special kind of bond with another atom, right? It all comes down to electronegativity. The more electronegative an atom is, the stronger it pulls on the shared electrons in a bond. Think of it like a tug-of-war, where the more electronegative atom wins!
So, when hydrogen is bonded to a really electronegative atom like oxygen, the electrons get pulled closer to the oxygen, making the hydrogen atom slightly positive. This creates a partial positive charge on the hydrogen. Now, this slightly positive hydrogen can form a weak bond, called a hydrogen bond, with another electronegative atom like oxygen or nitrogen in a nearby molecule.
It’s like a little magnetic attraction between the positive hydrogen and the negative oxygen or nitrogen. Think of it as the hydrogen atom being attracted to the opposite pole of a magnet.
Hydrogen bonds are weaker than regular chemical bonds, but they’re still very important. They play a huge role in holding molecules together, like in water, where hydrogen bonds are the reason water is a liquid at room temperature. They’re also important in proteins, DNA, and lots of other biological molecules!
But remember, for a hydrogen bond to form, the hydrogen atom has to be bonded to a really electronegative atom, like oxygen. That’s why hydrogen bonded to oxygen can form a stronger hydrogen bond than hydrogen bonded to carbon.
Now, let’s get a little deeper into this. Think of it this way: the more electronegative an atom is, the more it pulls on the electrons in the bond, making the hydrogen more positive and therefore more likely to form a hydrogen bond.
For example, oxygen is much more electronegative than carbon. This means the electrons in the oxygen-hydrogen bond will be pulled more strongly toward the oxygen, making the hydrogen more positive and more likely to form a hydrogen bond with another electronegative atom.
In contrast, the electrons in the carbon-hydrogen bond are not pulled as strongly toward the carbon, so the hydrogen is less positive and less likely to form a hydrogen bond.
You can think of it as a tug-of-war where the oxygen atom in the oxygen-hydrogen bond is like a strong wrestler pulling the electrons towards itself, making the hydrogen atom more likely to form a hydrogen bond. But the carbon atom in the carbon-hydrogen bond is like a weaker wrestler, so the hydrogen atom is less likely to form a hydrogen bond.
So, in conclusion, a hydrogen bond can only form when hydrogen is attached to a really electronegative atom, like oxygen, because the hydrogen becomes partially positive and can then interact with a nearby electronegative atom like oxygen or nitrogen.
See more new information: linksofstrathaven.com
Hydrogen Bonding: Always Present When Hydrogen Is?
The Basics of Hydrogen Bonding
So, what makes hydrogen bonding so special? Well, it all comes down to hydrogen, a little atom with a big personality. Hydrogen is super small and has only one proton. When it’s hanging out with a really electronegative atom like oxygen, nitrogen, or fluorine, it gets a little bit stripped of its electron. This makes the hydrogen atom slightly positive, like a tiny magnet with a positive charge. And the other atom, the one that’s hogging the electron, becomes slightly negative.
This difference in charge is what creates the hydrogen bond. The positive hydrogen atom gets attracted to the negative end of another molecule, and voila! Hydrogen bond! Think of it like magnets sticking together.
Hydrogen Bonding: Not Just a Fancy Name
Now, you might be thinking, “Why is hydrogen bonding such a big deal?” Well, it’s a big deal because it affects a lot of things in our world! It’s responsible for:
The high boiling point of water: Water molecules are incredibly good at hydrogen bonding, which is why water is a liquid at room temperature. Without hydrogen bonding, water would be a gas!
The structure of DNA: Hydrogen bonding is what holds the two strands of DNA together, like a zipper. This is super important because it allows DNA to store and transmit genetic information.
The properties of proteins: Hydrogen bonds are crucial for the folding and shape of proteins, which are responsible for all sorts of important functions in our bodies, like building and repairing tissues, fighting off infections, and carrying oxygen.
Hydrogen Bonding: A Bit More Detail
Here are some things to remember about hydrogen bonding:
It’s a relatively strong intermolecular force: It’s stronger than other intermolecular forces, like Van der Waals forces, but it’s not as strong as covalent bonds.
It’s highly directional: The hydrogen bond can only form in a specific direction, like a magnet that only sticks to a certain pole.
It’s important for many biological processes: As we mentioned, hydrogen bonding is crucial for the structure and function of DNA, proteins, and many other important biological molecules.
The Bottom Line
So, the next time you see water or hear about DNA, remember that hydrogen bonding is playing a crucial role! It’s a fundamental force in chemistry and biology, and it’s essential for life as we know it.
FAQs
Q: Is hydrogen bonding present in all molecules that contain hydrogen?
A: No, hydrogen bonding only occurs when hydrogen is directly bonded to a highly electronegative atom, like oxygen, nitrogen, or fluorine.
Q: What are some examples of hydrogen bonding in everyday life?
A: Water, DNA, proteins, and even the way your clothes stick together after you take them out of the dryer are all examples of hydrogen bonding in action!
Q: Why is hydrogen bonding so important for life?
A: Hydrogen bonding plays a crucial role in the structure and function of many biological molecules, including DNA, proteins, and carbohydrates. It helps to hold these molecules together and allows them to perform their specific functions.
Q: How can I learn more about hydrogen bonding?
A: You can find lots of information about hydrogen bonding in textbooks, online resources, and scientific journals. There are also many great videos and animations that can help you visualize how hydrogen bonding works.
I hope this has helped clear things up! If you have any more questions about hydrogen bonding, feel free to ask.
Hydrogen Bonding – Chemistry LibreTexts
A hydrogen bond is a weak type of force that forms a special type of dipole-dipole attraction which occurs when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of Chemistry LibreTexts
7.6: Hydrogen Bonding – Chemistry LibreTexts
Hydrogen bonding occurs when there is a significant amount of positive charge building up on a hydrogen atom. That happens because the Chemistry LibreTexts
Hydrogen bonding (video) | States of matter | Khan Academy
Hydrogen bonding is a special type of dipole-dipole interaction that occurs between the lone pair of a highly electronegative atom (typically N, O, or F) and the hydrogen atom Khan Academy
Hydrogen bonding | Definition, Examples, & Facts | Britannica
Hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; such a bond is weaker than an Britannica
Hydrogen Bond Definition and Examples – Science
A hydrogen bond is an attractive dipole-dipole interaction between a partially positive charged hydrogen atom in one molecule and a partially negative charged atom in the same or different molecule. As the Science Notes and Projects
Hydrogen Bond: Definition, Types, and Examples
A hydrogen bond is a type of chemical bond that involves the electrostatic attraction between a hydrogen atom and an atom containing a lone pair of electrons in a substance. In order for a hydrogen bond to occur, the Chemistry Learner
intermolecular bonding – hydrogen bonds – chemguide
Hydrogen bonding also occurs in organic molecules containing N-H groups – in the same sort of way that it occurs in ammonia. Examples range from simple molecules like CH 3 NH 2 (methylamine) to large molecules like chemguide
Hydrogen Bonds – Chemistry | Socratic
Answer: Hydrogen bonds are intermolecular forces; covalent and ionic bonds are intramolecular forces. Explanation: Ionic Bonds. Ionic bonds form when one atom Socratic
8.3 The Key Role of Hydrogen Bonding – American Chemical
A hydrogen bond is an electrostatic attraction between a H atom, which is bonded to a highly electronega-tive atom (O, N, or F), and a neighboring O, N, or F atom—either in American Chemical Society
Hydrogen Bonds In Water Explained – Intermolecular Forces
Hydrogen Bonding And Common Mistakes
A Level Chemistry Revision \”Hydrogen Bonding\”.
How To Tell Which Molecules Hydrogen Bond
Hydrogen Bonding (A-Level Chemistry)
Hydrogen Bonding In Water
Hydrogen Bonding | Chemistry
Link to this article: hydrogen bonding occurs whenever hydrogen is present.
See more articles in the same category here: https://linksofstrathaven.com/how