Let’s discuss the question: how many molecules are in 3na2so4. We summarize all relevant answers in section Q&A of website Linksofstrathaven.com in category: Blog Finance. See more related questions in the comments below.
How many molecules are in a molecule?
Molecule: group of two or more atoms held together by chemical bonds. So, minimum 2 atoms are required to form a molecule.
How many atoms does znso4?
It has one zinc atom, four oxygen atoms a one sulfur atom.
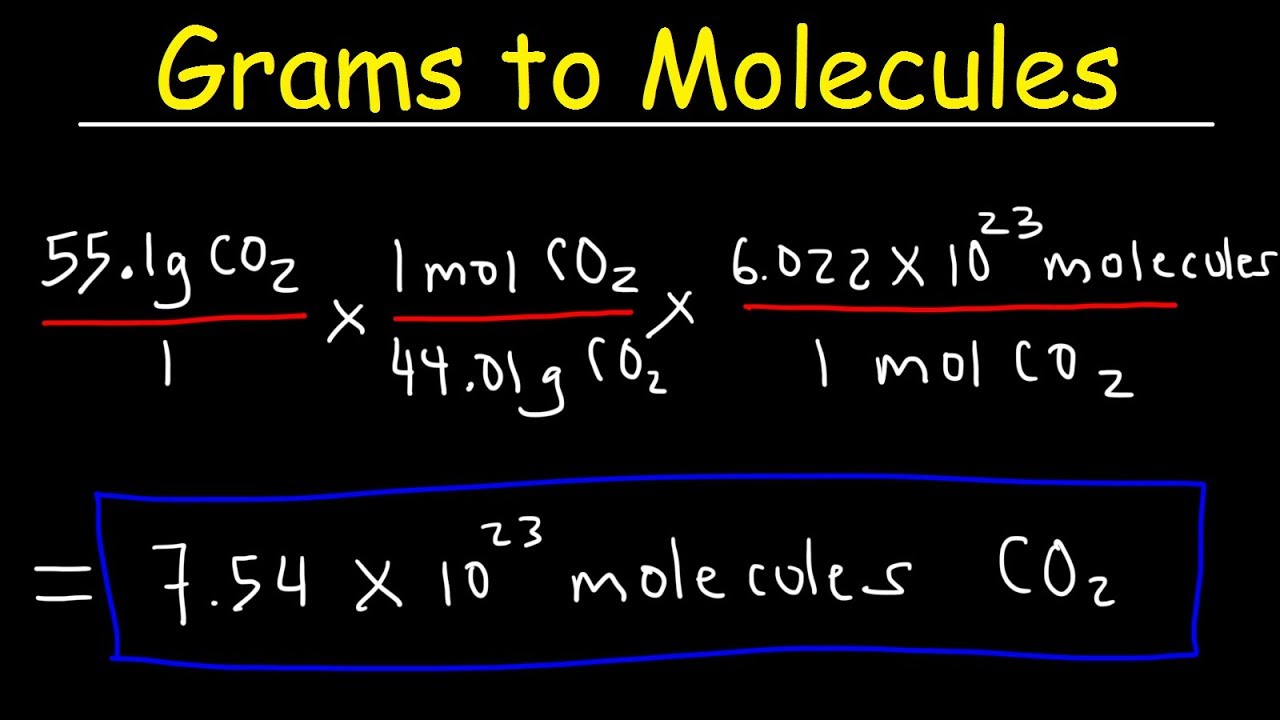
Grams to Molecules and Molecules to Grams Conversion
Images related to the topicGrams to Molecules and Molecules to Grams Conversion

How much molecules are in an atom?
If it is molecules, it’s 6.022 X 1023 of them. If it is atoms, it’s 6.022 x1023 atoms. If there are 2 atoms per molecule you need to double the number of moles. Each compound has its own, unique molar mass.
How many molecules are in 1.5 moles?
Solution. Hence, number of molecules in 1.5 moles of ammonia is 9.033 × 1023.
How many molecules are in a cell?
University of Toronto. “A cell holds 42 million protein molecules, scientists reveal.” ScienceDaily. ScienceDaily, 17 January 2018.
How many molecules are in 2 moles?
If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6H 6 molecules, or 3.011 × 10 23 C 6H 6 molecules.
How many atoms are in a cell?
Scientists estimate the average cell contains 100 trillion atoms. The number of atoms per cell is about the same as the number of cells in the body.
How many molecules are in each sample 19.3 g C8H10?
How many molecules are in each sample 19.3 g C8H10? Okay, now we can cancel grams with grams and moles with moles, so your answer should be 1.09 e plus 23 molecules of C8H10.
What are the elements of ZnSO4?
It contains a zinc(2+). Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as “white vitriol”.
How many zinc atoms are in ZnSO4?
The number of atoms of each element present is: Zinc (1 atom) Sulfur (1atom) Oxygen (4 atoms)
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

What is the molecular mass of ZnSO4?
How many molecules are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
How many atoms are there?
According to the US Department of Energy’s Jefferson Lab, the answer is: 133,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000. That answer comes from an estimation of the number of atoms in each of Earth’s elements, like Iron, Oxygen, Silicon, Magnesium, Sulfur … etc.
How many atoms are in 2 waters?
There are three atoms in a water molecule: an oxygen atom and two atoms of hydrogen, which are bonded together like small magnets. The number of atoms present within each molecule represents the second conversion factor. 4 hydrogen atoms and 2 oxygen atoms are found in two water molecules.
How many molecules is 3 moles?
A mole of anything contains 6.022×1023 individual items of that something. You have 3 moles, so there are 3×6.022×1023 oxygen molecules .
How many molecules are in 2 moles h2o?
One mole of H₂O contains 6.023 × 10²³ number of H₂O molecules. This is so because 1 mole of any substance contains the amount of 6.023 × 10²³ constituents in it, also known as the Avogadro number. Thus, 2 moles of H₂O will contain 2 × 6.023 × 10²³ number of H₂O molecules, that is 12.046 × 10²³ molecules of H₂O.
How many molecules are in a bacterial cell?
| Value | 2e+10 water molecules |
|---|---|
| Organism | Bacteria Escherichia coli |
| Reference | “Physical Biology of the Cell”, Rob Phillips, Jane Kondev and Julie Theriot (2009). Page 34 |
Is a molecule a cell?
Some cells are organisms unto themselves; others are part of multicellular organisms. All cells are made from the same major classes of organic molecules: nucleic acids, proteins, carbohydrates, and lipids.
Are there molecules in cells?
Cells are composed of water, inorganic ions, and carbon-containing (organic) molecules. Water is the most abundant molecule in cells, accounting for 70% or more of total cell mass.
How to find the Number of Atoms in a Molecule
Images related to the topicHow to find the Number of Atoms in a Molecule

How many molecules are in 4 mol mol of molecules?
The answer is 1.660538863127E-24. We assume you are converting between mole and molecule. You can view more details on each measurement unit: moles or molecule The SI base unit for amount of substance is the mole. 1 mole is equal to 6.0221415E+23 molecule.
How many molecules are there in 1 mole of CO2?
Mass of 1 mole (6.023 X 1023 molecules) of CO2 is about 44g.
Related searches
- what is the number of molecules in 3na2so4
- how many total atoms are present in 400 grams of na2so4
- how many elements are in 3na2so4
- number of molecules in 2znso4
- how many molecules are in h2o2
- how to find the number of molecules
- total atoms in 3na2so4
- 3na2so4 element
- how many molecules does 3na2so4 have
- 3na2so4 name
- k2co3 number of molecules
- how many molecules are in 2h2o
- how many total atoms are in 4ca3po42
- 3na2so4 chemical name
- what is 3na2so4
Information related to the topic how many molecules are in 3na2so4
Here are the search results of the thread how many molecules are in 3na2so4 from Bing. You can read more if you want.
You have just come across an article on the topic how many molecules are in 3na2so4. If you found this article useful, please share it. Thank you very much.
