How many lone pairs does O3 have?
Ozone has a unique structure with a central oxygen atom bonded to two other oxygen atoms. One of these bonds is a double bond, while the other is a single bond. Lone pairs are pairs of electrons that are not involved in bonding.
The oxygen atom with the double bond has two lone pairs. The oxygen atom with the single bond has three lone pairs. This gives us a total of five lone pairs. Lone pairs play a crucial role in determining the shape and reactivity of molecules.
Here’s a more detailed explanation:
The Central Oxygen: This oxygen atom forms a double bond with one of the other oxygen atoms. Double bonds consist of two shared electron pairs. Since oxygen has six valence electrons, after forming the double bond, it has two remaining electrons, forming one lone pair.
The Terminal Oxygen with a Single Bond: This oxygen atom forms a single bond with the central oxygen. A single bond involves one shared electron pair. Since oxygen has six valence electrons, after forming the single bond, it has four remaining electrons, forming two lone pairs.
The Terminal Oxygen with a Double Bond: This oxygen atom forms a double bond with the central oxygen. Double bonds consist of two shared electron pairs. Since oxygen has six valence electrons, after forming the double bond, it has two remaining electrons, forming one lone pair.
Therefore, ozone (O3) has a total of five lone pairs. This is different from what you might initially expect, as it doesn’t have six lone pairs as previously stated. The structure and bonding within ozone contribute to its unique properties and reactivity.
How many single bonds are in O3?
Let’s break down the structure of ozone to understand how many single bonds it has. You can think of ozone as a bent molecule with a central oxygen atom connected to two other oxygen atoms. One of these connections is a double bond, while the other is a single bond.
Think of it this way: a double bond is like two pairs of hands holding onto each other tightly, while a single bond is like one pair of hands. This double bond is between the central oxygen atom and one of the outer oxygen atoms. The other outer oxygen atom is connected to the central oxygen atom by a single bond.
Now, here’s the cool part: ozone is a bit tricky because it has resonance structures. This means that the double bond can actually shift between the two outer oxygen atoms. This shifting makes the ozone molecule more stable and gives it a bit of extra reactivity.
The single bond between the central oxygen atom and one of the outer oxygen atoms is a key feature of ozone. This bond, along with the double bond and the overall bent shape of the molecule, contributes to ozone’s unique properties, including its ability to absorb ultraviolet radiation in the atmosphere.
How to find lone pairs?
You’ve already counted up the valence electrons on your central atom (that’s Step 1), and you’ve figured out how many valence electrons are being used in bonds with other atoms (that’s Step 2). Now, we’re ready to find the lone pairs!
To do this, simply subtract the number of valence electrons used in bonds from the total number of valence electrons on the central atom. The difference tells you how many valence electrons are left over. These leftover valence electrons are your lone pairs!
For instance, if your central atom has six valence electrons and four of them are used in bonds, you have two valence electrons left over. These two valence electrons form one lone pair.
Think of it this way:
* Each bond uses two valence electrons, one from each atom involved.
Lone pairs are those extra valence electrons that aren’t involved in forming bonds. They hang out on the central atom, giving it a unique shape and reactivity.
Let’s try a real-world example. Take water (H2O). Oxygen (O) is the central atom. Oxygen has six valence electrons. Each hydrogen (H) atom contributes one valence electron to the bond. So, there are two valence electrons used in bonds. Subtracting the two valence electrons used in bonds from the six valence electrons on the oxygen atom leaves us with four valence electrons – that’s two lone pairs on oxygen!
Finding lone pairs is crucial because they play a big role in determining a molecule’s shape and its chemical behavior. So, keep this handy trick in mind – subtract to find the lone pairs and you’ll be well on your way to understanding how molecules work!
Why does O3 have 3 bonds?
The central oxygen atom in ozone is sp2 hybridized, meaning it has three hybrid orbitals that are used to form covalent bonds. These hybrid orbitals are created by mixing one *s* orbital and two *p* orbitals, resulting in three equivalent orbitals that are arranged in a trigonal planar geometry.
This arrangement allows the central oxygen to form a single bond with one oxygen atom and a double bond with the other oxygen atom, giving ozone a total of three covalent bonds.
To understand this better, let’s visualize the structure. Imagine the central oxygen atom as the center of a triangle. Each corner of the triangle represents a bond:
One single bond is formed by the central oxygen sharing one electron with one of the outer oxygen atoms.
One double bond is formed by the central oxygen sharing two electrons with the other outer oxygen atom.
This double bond is actually a combination of two types of bonds: a sigma bond and a pi bond. The sigma bond is a direct overlap of orbitals, while the pi bond is a sideways overlap of orbitals above and below the plane of the molecule.
The sp2 hybridization of the central oxygen atom in ozone is responsible for the unique bent structure of the molecule and the three covalent bonds that hold it together. It’s this structure that gives ozone its distinctive reactivity and its role in the Earth’s atmosphere.
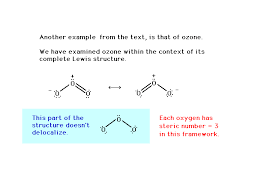
Why are two bonds in O3 equal?
Resonance is a way of describing the bonding in molecules where the electrons are delocalized, meaning they are not fixed to a particular atom or bond. In the case of ozone, the electrons involved in the bonding are spread out over all three oxygen atoms. This delocalization gives both O-O bonds a partial double bond character, which is why they are equal in length.
Imagine the O-O bonds as constantly shifting back and forth between a single bond and a double bond. This constant “shifting” averages out the bond lengths, resulting in two equal bonds.
Let’s delve deeper into this concept:
Imagine a single ozone molecule, but let’s simplify it by drawing it in a linear form for now. You could draw two possible Lewis structures for this molecule. In one structure, the central oxygen atom would have a double bond to one of the terminal oxygen atoms and a single bond to the other. In the other structure, the central oxygen would have a single bond to the first terminal oxygen and a double bond to the second terminal oxygen.
However, in reality, the molecule is not stuck in one of these specific Lewis structures. Instead, it exists as a hybrid of both structures, with the electrons in the bonds spread out over all three oxygen atoms. This phenomenon is called resonance.
Resonance contributes to the stability of the ozone molecule. While the Lewis structures themselves are just representations, the actual ozone molecule is a blend of these structures. Think of it as a photograph taken with a long exposure – the image is not static but shows the blur of the movement, capturing the essence of the molecule’s dynamic nature. This is the key to understanding why the O-O bonds in ozone are equal. They share the electron density, making them effectively the same.
It’s important to note that resonance doesn’t mean the electrons are “jumping” between atoms. It’s a way of describing how the electrons are distributed in a way that cannot be represented by a single Lewis structure. Resonance is a powerful concept in chemistry, helping us understand the behavior of molecules like ozone, and explaining why their bonds are equal even though they might appear different on paper.
Why is O3 not two double bonds?
You might think that drawing ozone with two double bonds would seem like a good idea. But, the central oxygen atom would have more than eight electrons, violating the octet rule. This rule states that atoms want to have eight electrons in their outer shell to achieve stability.
Think of it this way: Oxygen has six electrons in its outer shell. If you draw two double bonds, that would give the central oxygen atom ten electrons! That’s too many!
So, what’s the solution? Ozone actually has a resonance structure. This means that the electrons in the bonds are delocalized, meaning they are not fixed in one position but spread out over the entire molecule. Instead of two double bonds, ozone has one double bond and one single bond, with a partial double bond character. This structure allows the electrons to be distributed evenly, satisfying the octet rule for all the oxygen atoms.
Remember, elements in the second row of the periodic table, like oxygen, tend to follow the octet rule. This is because they only have s and p orbitals in their outer shell, which can hold a maximum of eight electrons. Elements below the second row have access to d orbitals, which allows them to accommodate more than eight electrons.
Think of it like having a small house (s and p orbitals) that can only fit eight people (electrons) comfortably. But, larger houses (elements below the second row) have additional rooms (d orbitals) where more people (electrons) can live. So, those elements can have expanded octets.
See more here: How Many Single Bonds Are In O3? | How Many Lone Pairs Are There In O3
How many lone pairs does O3 (ozone) have?
Ozone has a central oxygen atom bonded to two other oxygen atoms. The central oxygen atom has one lone pair of electrons. Each of the outer oxygen atoms has two and three lone pairs of electrons.
Here’s how we can break down why this is the case:
1. Valence Electrons: Oxygen has six valence electrons.
2. Lewis Structure: The Lewis structure for ozone shows one double bond and one single bond between the oxygen atoms.
3. Central Oxygen: The central oxygen atom forms two bonds (one double and one single) and has one lone pair.
4. Outer Oxygen Atoms: The outer oxygen atoms form one bond (either a double or a single) and have three lone pairs.
Let’s visualize this:
Central Oxygen: The central oxygen atom forms two bonds, leaving two electrons to form a lone pair.
Outer Oxygen Atoms: The outer oxygen atoms each have one bond. To satisfy the octet rule, they need eight electrons in their valence shell, so they have three lone pairs each.
So, to summarize:
Central Oxygen: One lone pair.
Outer Oxygen Atoms: Two and three lone pairs each.
Understanding how to determine lone pairs helps us understand the structure, reactivity, and properties of molecules like ozone.
How many lone pairs does O1 have?
The molecule you’re describing is ozone, O3. It has a unique structure with a central oxygen atom (O2) bonded to two other oxygen atoms (O1 and O3).
O1 has two lone pairs of electrons. This is because oxygen has six valence electrons, and it forms one single bond with the central oxygen atom. After forming the bond, O1 still has four electrons left, which are arranged as two lone pairs.
Here’s a more detailed explanation:
1. Oxygen’s Valence Electrons: Oxygen has six valence electrons, which are the electrons in its outermost shell.
2. Bond Formation:O1 forms a single bond with the central oxygen atom (O2). This bond involves the sharing of one electron from each atom.
3. Remaining Electrons: After forming the bond, O1 has four electrons remaining.
4. Lone Pairs: These four remaining electrons are arranged as two lone pairs on O1.
Let’s look at the other oxygen atoms:
O2 (the central oxygen atom) has one lone pair. It forms two bonds, one with O1 and one with O3, leaving one lone pair.
O3 has three lone pairs. Like O1, it forms one bond with the central oxygen atom (O2), leaving three lone pairs.
Remember, lone pairs are important because they influence the shape of molecules and their reactivity. The presence of lone pairs on the oxygen atoms in ozone contributes to its bent molecular geometry.
How many lone pairs does NH3 have?
We know that nitrogen has five valence electrons, and hydrogen has one. In NH3, nitrogen forms three single bonds with three hydrogen atoms, leaving one lone pair of electrons on the nitrogen atom. So, the answer to your question is one.
Here’s a breakdown to help you visualize it:
Nitrogen needs three electrons to complete its octet (eight electrons in its outermost shell).
Hydrogen only needs one electron to complete its duet (two electrons in its outermost shell).
Nitrogen shares one electron with each of the three hydrogen atoms, forming three single bonds.
* Since nitrogen has five valence electrons and uses three for bonding, it has two electrons remaining.
* These two electrons form a lone pair on the nitrogen atom.
You can picture this as the nitrogen atom being in the center of a pyramid with the hydrogen atoms at the corners and the lone pair pointing up. This arrangement is called trigonal pyramidal, and it’s because of the lone pair that ammonia is a polar molecule. The lone pair pushes the hydrogen atoms down, giving it that pyramidal shape.
The lone pair of electrons on the nitrogen atom in ammonia makes it a Lewis base, meaning it can donate its electron pair to form a coordinate covalent bond.
What is the best Lewis structure of O3?
Ozone is a fascinating molecule made up of three oxygen atoms. To understand its Lewis structure, we need to consider the valence electrons. Each oxygen atom has six valence electrons, and with three atoms, we have a total of 18 valence electrons to work with.
When drawing the Lewis structure, we aim for the most stable arrangement, which involves minimizing formal charges. We start by placing the oxygen atoms in a line, with the central oxygen atom bonded to the other two. Then, we distribute the valence electrons as lone pairs and bonding pairs to achieve a stable octet around each oxygen atom.
Here’s the breakdown:
Central Oxygen: The central oxygen atom forms two double bonds with the other two oxygen atoms. This gives it four electrons involved in bonding and two lone pairs, resulting in a stable octet.
Terminal Oxygen Atoms: Each terminal oxygen atom forms one double bond with the central oxygen atom and has three lone pairs. This also gives them a stable octet.
This arrangement satisfies the octet rule for all three oxygen atoms and minimizes formal charges. Therefore, this Lewis structure is the most stable and the best representation of ozone.
Remember, we want to ensure that all the valence electrons are accounted for. If we try to add more lone pairs to form additional bonds, it would violate the octet rule for some of the oxygen atoms, leading to an unstable structure. That’s why the structure with two double bonds and three lone pairs on the terminal oxygen atoms is the best Lewis structure for ozone.
See more new information: linksofstrathaven.com
How Many Lone Pairs Are There In O3 | How Many Lone Pairs Does O3 Have?
Ozone is a fascinating molecule! It’s made up of three oxygen atoms, and it plays a crucial role in our atmosphere, protecting us from harmful UV radiation. But when it comes to lone pairs, we need to look at the structure of ozone to understand.
Ozone’s Lewis structure shows that the central oxygen atom has one double bond to one of the other oxygen atoms and a single bond to the third oxygen atom. That means the central oxygen atom has two lone pairs of electrons.
The Breakdown
Here’s how we can visualize this:
Central Oxygen Atom: It’s bonded to two other oxygen atoms. It has one double bond and one single bond.
Lone Pairs: The central oxygen atom has two lone pairs.
So, the answer to your question is that there are two lone pairs of electrons on the central oxygen atom in the ozone molecule.
Now, let’s talk about why lone pairs matter.
Lone Pairs and Molecular Geometry
Lone pairs affect the molecular geometry of a molecule. In ozone, the presence of those two lone pairs on the central oxygen atom pushes the other two oxygen atoms away from each other, resulting in a bent or V-shaped molecular geometry. This bent shape is important because it influences how ozone interacts with other molecules.
Ozone’s Role in Chemistry
Ozone is a powerful oxidizer, which means it can easily take electrons from other molecules. This property makes it an important component in various chemical reactions. In the atmosphere, ozone plays a vital role in absorbing harmful ultraviolet radiation from the sun, shielding life on Earth.
Other Important Points to Remember
Valence Electrons: Each oxygen atom has six valence electrons.
Hybridization: The central oxygen atom in ozone undergoes sp² hybridization.
Bond Angles: The bond angles in ozone are approximately 116.8°.
Let’s Look at Ozone’s Lewis Structure:
Here’s a visual representation of ozone’s Lewis structure, which helps you see the lone pairs:
“`
O
||
:O – O:
“`
Key Points to Remember:
Two lone pairs are present on the central oxygen atom in ozone (O3).
* Lone pairs influence the molecular geometry, resulting in a bent or V-shaped structure.
* Ozone is a strong oxidizer due to the presence of these lone pairs.
I hope this explanation has shed some light on the lone pairs in ozone and their importance!
FAQs about Ozone and Lone Pairs
Q: What is a lone pair of electrons?
A: A lone pair of electrons is a pair of electrons that are not involved in bonding with other atoms. They reside in the outermost shell of an atom and are not shared with other atoms.
Q: How do lone pairs affect the shape of a molecule?
A: Lone pairs repel bonding pairs of electrons, causing them to spread out further. This repulsion affects the bond angles and overall molecular geometry of the molecule. In ozone, the two lone pairs on the central oxygen atom cause the molecule to bend into a V-shape.
Q: Why is ozone important?
A: Ozone plays a crucial role in the Earth’s atmosphere, absorbing harmful ultraviolet radiation from the sun. This absorption protects life on Earth from the damaging effects of UV radiation. However, ozone can also be harmful at ground level, contributing to smog and respiratory problems.
Q: What are some other examples of molecules with lone pairs?
A: Many molecules have lone pairs, including water (H2O), ammonia (NH3), and sulfur dioxide (SO2). These lone pairs influence the shape and reactivity of these molecules.
Q: Is there anything else I should know about ozone?
A: Ozone is a very reactive molecule and can be harmful to humans and the environment. Ozone depletion, which is the thinning of the ozone layer, is a serious environmental concern. It’s important to understand the role of ozone in the environment and to protect it.
O3 Lewis Structure: Drawings, Hybridization, Shape, Charges,
The Lewis structure of O3, also known as ozone, consists of three oxygen atoms bonded together. Each oxygen atom is connected to the central oxygen atom by techiescience.com
O3 Lewis Structure, Molecular Geometry,
Molecular Geometry of O3. To find out the molecular geometry of ozone we need to check the VSEPR theory model. How to proceed with this? At the very beginning, you have to check the terminal atoms. Techiescientist
How to Determine the Number of Lone Pairs
In general, there are two approaches you can use to determine the number of lone pairs. The first one, which is also what you should eventually aim for, is to learn the common bonding patterns of the elements in the Chemistry Steps
Ozone (O3) Lewis Structure – Steps of Drawing – Learn Chemistry
Lone pairs on atoms. After determining the center atom and sketch of O 3 molecule, we can start to mark lone pairs on atoms. Remember that, there are total of nine electron chemistryscl.com
O3 Lewis Structure – Chemistry Steps
We now have 14 valence electrons left, and since the middle oxygen has two bonds while the terminal oxygens have one, we add them to terminal oxygens as two pairs: Next, Chemistry Steps
What is the Lewis structure of ozone (#O_3#)? – Socratic
Explanation: Simple VESPER requires that we distribute 3 ×6 = 18 valence electrons across 3 centres: O = O+ − O−. From the left, O1, has TWO lone pairs; O2 Socratic
Ozone (O3) – Ozone Structure, Molecular Mass, Properties
How many lone pairs are in Ozone? In the ozone the Oxygen containing double bond has two lone pairs; oxygen containing positive charge has one lone pair and the oxygen BYJU’S
Lewis Structure of O3 (With 6 Simple Steps to Draw!) – Knords
Lewis structure of O3 (Ozone) contains one double bond and one single bond between both the Oxygen (O) atoms. The central Oxygen atom has one lone pair, Knords Learning
O3 Lewis Structure – Ozone | Video Summary and Q&A | Glasp
To draw the Lewis structure of ozone (O3), calculate the total number of valence electrons, determine the number of lone pairs on the central oxygen atom, and Glasp
O3 Lewis Structure – How To Draw The Dot Structure For O3
O3 Lewis Structure – Ozone
How To Calculate Bond Pair And Lone Pair Of Electrons? Easy Trick
Is Ozone O3 Polar ? (Yes)
How To Identify The Number Of Lone Pairs On An Atom Using Formal Charge
Number Of Lone Pairs And Bonding Pairs For O2
Resonance Structures Of O3, Ozone
Link to this article: how many lone pairs are there in o3.
See more articles in the same category here: https://linksofstrathaven.com/how
