Let’s discuss the question: how many atoms are in a mole of gold. We summarize all relevant answers in section Q&A of website Linksofstrathaven.com in category: Blog Finance. See more related questions in the comments below.

How many atoms are in 2 moles of gold?
How many atoms are there in 2 moles of gold Au )? We know we have 2 moles of Au, so multiply 6.02 x 2. Answer is 12.04 x 10^23 atoms of Au.
How many moles are in gold?
The molar mass of gold = 196.96657 g/m. The molar mass of gold is defined as the mass of 1 mole of gold atoms – typically specified in grams. Therefore the molar mass of gold is stated in units of grams/mole or g/m. It is also frequently called the Molecular Weight of Gold.
Number of Atoms in a Mole
Images related to the topicNumber of Atoms in a Mole

How many atoms are there in a gold molecule?
There are 6.022 X 1023 atoms in one mole of gold.
How many atoms are in a mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
How many atoms of gold are in 3.5 moles of gold?
1.07×1022 atoms.
How many atoms are in 4.5 moles?
1 Expert Answer
A mole of anything has 6.022 x 1023 items in it. 4.5 moles of copper has (4.5)(6.022 x 1023) = 2.7 x 1024 atoms.
What is the mole of water?
For example: H2O has two hydrogens and one oxygen. The atomic mass of hydrogen is 1.0078 and the atomic mass of oxygen is 15.999, so the mass of a mole of water is 2 x 1.0078 + 15.999. Therefore, a mole of water, or 6.022 x 1023 molecules, weighs 18.0146 grams.
How many atoms are in three moles?
Explanation: 1 mole of O₂ contains 6.022 × 10²³ atoms. Thus, 3 moles O₂ would contain 3 × 6.022 × 10²³ atoms. Therefore, 3 moles of O₂ contain 18.066 × 10²³ atoms.
What is mole of gold?
For example, gold has an atomic weight of 196.967 amu, so one mole of gold has a mass of 196.967 grams. For a substance that is composed of more than one kind of atom, one adds up the atomic weights of the individual atoms for the chemical unit that makes up that substance.
How many molecules are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
How many atoms are in a mole of iron?
We know that 1 mole of iron element contains 6.022×1023 atoms of iron. Thus, a piece of iron metal having a mass of 2.8 grams contains 3.011×1022 atoms of iron.
How many atoms of gold are there in one ounce of gold?
One troy ounce is 31.1g. So that comes to $450/31.1= $14.47 per gram. The age of the universe is 1.4×1010 years = 4.418×1017 seconds. Since the atomic weight of gold is 197 and Avogadro’s number is 6.02×1023, 4.418×1017 atoms of gold = (4.418×1017/6.02×1023)x197grams = .
Moles To Atoms Conversion – Chemistry
Images related to the topicMoles To Atoms Conversion – Chemistry
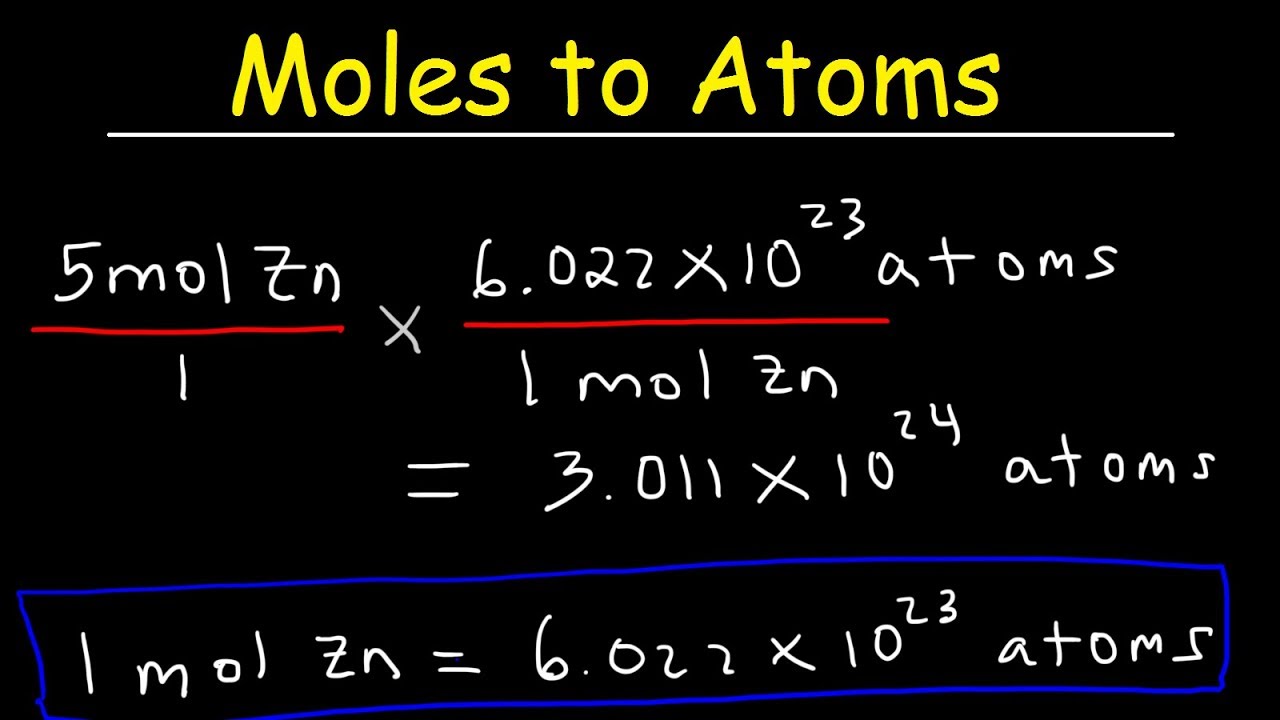
How many atoms are in 2 moles?
If 1 mole of atoms is 6.02 x 1023 atoms, then 2 moles of atoms would be equal to 1.20 x 1024 atoms.
How many atoms are in a mole of water?
one mole of water contains 6.02 x 1023 MOLECULES of water.
But each molecule of water contains 2 H and 1 O atom = 3 atoms, so there are approximately 1.8 x 1024 atoms in a mole of water.
How do you find atoms from moles?
Avogadro’s number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc. To convert from moles to atoms, multiply the molar amount by Avogadro’s number. To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
How do you find atoms in gold?
Calculate the moles of gold by dividing the given mass by its molar mass, 196.966569 g/mol (atomic weight on periodic table in g/mol). Multiply the calculated mol Au times 6.022×1023atoms1mol .
How many atoms are in 2.5 moles?
2.5⋅mol×6.022×1023⋅mol−1=15.06×1024⋅nickel atoms .
How many atoms of gold are there in 2.5 moles?
There are 15.055 X 1023 atoms of gold in a sample of 2.5 mol of Au, also known as gold.
How many atoms are in five moles?
Therefore, 5 moles of carbon contains 3.011 × 10²⁴ atoms.
How many atoms are in 4 moles helium?
– So, the no of atoms in 4 moles of helium is 2.4088 × 10²⁴.
How many atoms are in a mol of CU?
The relation between molecular (formula) mass and molar mass Page 4 4 • To obtain one mole of copper atoms (6.02 x 1023 atoms), weigh out 63.55 g copper. The molar mass (M) of a substance is the mass of one mole of entities (atoms, molecules, or formula units) of the substance.
Why is a mole 6.022 x10 23?
The MOLE (mol) is a unit of measurement that is the amount of a pure substance containing the same number of chemical units (atoms, molecules etc.) as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.022 X 1023).
Calculate the number of atoms of Gold in 1g of Gold? (Atomic mass:- 197g/mol)
Images related to the topicCalculate the number of atoms of Gold in 1g of Gold? (Atomic mass:- 197g/mol)

Can moles be brown?
Color and texture. Moles can be brown, tan, black, blue, red or pink. They can be smooth, wrinkled, flat or raised.
Which animal is mole?
Moles are small, burrowing mammals. Their eyes are poorly developed, but what they lack in sight, they make up for in their sense of touch. All moles have very sensitive snouts and long, clawed digits that they use to dig tunnels.
Related searches
- how many atoms are in 1 mole of gold
- what is the mass of 1 mole of gold
- how many atoms are present in a 0.0254 mole sample of gold
- how many atoms are present in a 0.600-mole sample of gold
- how many atoms are in 1.00 moles of he
- how many atoms are present in a 0.900-mole sample of gold
- how many atoms are present in a 0 300 mole sample of gold
- how many atoms are present in a 0.600 mole sample of gold
- how many atoms are in 1 00 moles of he
- how many atoms are present in a .300 mole sample of gold
- how many atoms are present in a 0 900 mole sample of gold
- how many atoms are present in a 0 600 mole sample of gold
- what is the mass of 1 mole of gold?
- atoms of gold to moles
- how many particles are there in 1 mole of water
- how many atoms are in 3 moles of gold
- how many atoms are contained in a 0.00250 mol sample of gold
- how many atoms or molecules are present in 1 mole
- how many atoms are present in a 0.300-mole sample of gold
Information related to the topic how many atoms are in a mole of gold
Here are the search results of the thread how many atoms are in a mole of gold from Bing. You can read more if you want.
You have just come across an article on the topic how many atoms are in a mole of gold. If you found this article useful, please share it. Thank you very much.
