Why is aniline less basic than methylamine?
The key lies in the electron density around the nitrogen atom. A higher electron density means the nitrogen is more likely to grab a proton (H+), making it more basic.
In aliphatic amines like methylamine, the alkyl groups have an inductive effect. This means they push electron density towards the nitrogen atom, increasing its basicity.
Aniline, on the other hand, has a benzene ring attached to the nitrogen. This ring is a conjugated system, meaning the electrons in the ring are spread out over the entire molecule. This delocalization of electrons reduces the electron density on the nitrogen atom in aniline.
Think of it like this: In methylamine, the nitrogen has a concentrated “pool” of electrons. In aniline, the electrons are spread out like a “thin film” over the entire ring. This “thin film” makes the nitrogen atom less available to grab a proton, hence its lower basicity.
In short, the conjugation in aniline reduces the electron density on the nitrogen atom, making it less basic than methylamine.
Let’s break down the conjugation aspect a bit more.
You can think of the benzene ring as a continuous circuit of electrons. These electrons are constantly moving, creating a delocalized “sea” of electrons. The lone pair of electrons on the nitrogen atom in aniline is part of this “sea”.
When the lone pair participates in this conjugation, it becomes less available for bonding with a proton. This is because the electrons are now spread out over the entire ring, rather than being concentrated on the nitrogen atom.
Think of it like this: if you have a bucket of water, it’s easy to pour it out all at once. But if you spread the water out over a large surface, it’s much harder to collect it all back into the bucket. Similarly, if the electrons on the nitrogen atom are spread out over the benzene ring, it’s harder for them to participate in a reaction to grab a proton.
This conjugation effect is a powerful force in organic chemistry and plays a crucial role in determining the properties of many molecules.
Why is methylamine more basic?
Let’s break this down further.
+I effect refers to the tendency of a group to donate electrons to the rest of the molecule. The CH3 group is an electron-donating group, which means it pushes electron density towards the nitrogen atom in methylamine. This makes the nitrogen atom more negatively charged and thus more likely to attract a proton, making methylamine a stronger base.
On the other hand, the -I effect of the benzene ring pulls electron density away from the nitrogen atom in aniline. This makes the nitrogen atom less negatively charged and less likely to attract a proton, making aniline a weaker base. The R effect of the benzene ring, which refers to the resonance effect, also contributes to the decrease in electron density on the nitrogen atom. This effect arises from the delocalization of electrons in the benzene ring, which further weakens the basicity of aniline.
To summarize, the difference in basicity between methylamine and aniline is due to the contrasting effects of the CH3 group and the benzene ring on the electron density of the nitrogen atom. The +I effect of the CH3 group makes methylamine more basic, while the -I and R effects of the benzene ring make aniline less basic.
Why is p-methylaniline more basic than aniline?
It’s all about the electron-donating effects of the methyl group! When a methyl group is attached to the para position of aniline, it has a +I effect, meaning it pushes electron density towards the nitrogen atom. This makes the nitrogen atom more electron-rich and, therefore, more likely to accept a proton, increasing its basicity.
Here’s a breakdown of why:
Aniline has a lone pair of electrons on the nitrogen atom, making it a base. However, the lone pair is partially delocalized into the benzene ring due to resonance, which reduces its availability for protonation, making aniline less basic than simple aliphatic amines.
p-methylaniline has a methyl group at the para position. The methyl group is electron-donating due to its +I effect. This effect pushes electron density towards the nitrogen atom, increasing the electron density on the nitrogen atom and making it more available for protonation. This effect outweighs the delocalization effect, making p-methylaniline more basic than aniline.
m-methylaniline also has a methyl group but at the meta position. While the methyl group still has a +I effect, it is less effective at donating electron density to the nitrogen atom due to the distance and the lack of direct conjugation with the benzene ring. Therefore, m-methylaniline is less basic than p-methylaniline but still more basic than aniline.
o-methylaniline is a bit more complex. The methyl group at the ortho position might be expected to increase the basicity due to its +I effect. However, the ortho effect comes into play, where the steric hindrance caused by the methyl group reduces the availability of the lone pair for protonation. This makes o-methylaniline less basic than aniline.
In essence, the electron-donating effect of the methyl group in p-methylaniline enhances the availability of the lone pair on the nitrogen atom, making it more basic than aniline.
Why aniline is less basic than ethylamine?
Aniline has a phenyl group attached to its nitrogen atom. This phenyl group is an electron-withdrawing group. This means it pulls electron density away from the nitrogen atom, making it less likely to accept a proton (H+).
Ethylamine, on the other hand, has an ethyl group attached to its nitrogen. This ethyl group is an electron-donating group. It pushes electron density towards the nitrogen atom, making it more likely to accept a proton and therefore more basic.
Think of it this way: Aniline’s nitrogen is like a magnet that’s lost some of its power because of the phenyl group, while ethylamine’s nitrogen is like a magnet with extra strength thanks to the ethyl group.
Here’s a more detailed explanation of how the phenyl group affects aniline’s basicity:
The phenyl group in aniline is a resonance-stabilized system. This means that the electrons in the benzene ring can delocalize (spread out) across the entire ring. This delocalization includes the lone pair of electrons on the nitrogen atom. As these electrons are delocalized into the ring, they become less available to form a bond with a proton. This effect, known as resonance effect, significantly reduces the electron density on the nitrogen atom, making aniline a much weaker base compared to ethylamine.
Imagine the nitrogen’s lone pair as a ball. In ethylamine, the ball is free to roll around, making it easy to grab. In aniline, the ball is stuck in a spinning wheel because of the phenyl group’s resonance. It’s much harder to snatch that ball from the spinning wheel. This makes aniline less basic compared to ethylamine.
Why aniline is less basic than aliphatic?
Aniline’s lone pair is less available for donation. This is because the lone pair of electrons on the nitrogen atom in aniline is involved in resonance with the aromatic ring. Resonance delocalizes the electron density, making the nitrogen atom less likely to accept a proton. In contrast, aliphatic amines don’t have this resonance effect. Their lone pair is more readily available for donation.
Think of it this way: The aromatic ring in aniline acts like a big, electron-hungry sponge. It draws electron density away from the nitrogen, making it less likely to grab a proton and become positively charged.
The +I effect in aliphatic amines makes them stronger bases. The +I effect is an inductive effect where electron density is pushed towards the nitrogen atom. This increased electron density makes the nitrogen atom more basic, as it’s more willing to donate its lone pair.
Let’s go a bit deeper into the concept of resonance and its impact on aniline’s basicity.
Imagine the lone pair on the nitrogen atom of aniline as a lone electron cloud. This cloud isn’t stuck on the nitrogen; it’s constantly moving and overlapping with the pi electron cloud of the benzene ring. This overlapping creates a delocalized electron system, which means the electron density is spread out over the entire ring.
Since the electron density is distributed, the nitrogen atom in aniline has a lower electron density compared to the nitrogen atom in an aliphatic amine. This reduced electron density makes it less likely to attract a proton and form a positive charge.
In simpler terms, the electron cloud is like a puddle of water. If the puddle is spread out, it’s shallower. In aniline, the electron cloud is spread out over the entire aromatic ring, making it less concentrated around the nitrogen atom. This “shallow” electron cloud is less likely to attract a proton.
On the other hand, aliphatic amines have a more localized electron cloud around the nitrogen atom. This concentrated electron cloud is like a deep puddle, making it more likely to attract a proton.
Key takeaway: The ability of a molecule to donate its lone pair of electrons determines its basicity. Aniline’s resonance effect reduces its ability to donate its lone pair, making it less basic than aliphatic amines, which have a stronger electron-donating effect.
Which is more basic than aniline?
Let’s break down why the +I effect makes benzyl amine more basic than aniline. The +I effect is an inductive effect where electron density is pushed through a sigma bond. In benzyl amine, the benzyl group has a +I effect because the carbon atoms in the benzyl group are more electronegative than the hydrogen atoms attached to them. This difference in electronegativity causes the electrons in the C-H bonds to be slightly shifted towards the carbon atoms, creating a partial negative charge on the carbon atoms and a partial positive charge on the hydrogen atoms. This partial positive charge on the hydrogen atoms is then transmitted through the sigma bond to the nitrogen atom in the amine group. The increased electron density on the nitrogen atom makes it more likely to attract a proton, thus increasing the basicity of the molecule.
In contrast, aniline has a phenyl group attached to the nitrogen atom. The phenyl group is an electron-withdrawing group, meaning it pulls electron density away from the nitrogen atom. This makes the nitrogen atom less likely to accept a proton, decreasing its basicity.
Think of it this way: the benzyl group is like a cheerleader for the nitrogen atom, boosting its electron density and making it more attractive to protons. The phenyl group, on the other hand, is like a grumpy neighbor, pulling electron density away from the nitrogen atom and making it less attractive to protons.
See more here: Why Is Methylamine More Basic? | Why Methylamine Is More Basic Than Aniline
Why is methyl amine more basic than aniline?
The key difference lies in the electron density around the nitrogen atom. In methyl amine, the nitrogen has a high concentration of electrons, making it more likely to accept a proton and act as a base.
In aniline, however, the nitrogen’s electrons are spread out across the aromatic ring through a process called resonance. This delocalization of electrons reduces the electron density on the nitrogen, making it less basic.
Think of it like this: In methyl amine, the nitrogen is like a magnet, strongly attracting protons. But in aniline, the nitrogen’s magnetism is weakened because its electrons are busy spreading out in the ring.
Here’s a simplified analogy: imagine methyl amine is a lone wolf, focused on attracting a proton. Aniline, on the other hand, is part of a pack, sharing its electrons with the ring, leaving it less eager to attract a proton.
This difference in electron density explains why methyl amine is a stronger base than aniline.
Why is ethyl amine more basic than ammonia?
Think of it this way: alkyl groups are like little pushers, they push electron density towards the nitrogen atom. This makes the nitrogen in ethyl amine more negatively charged and therefore more attracted to a proton (H+). The stronger the attraction to a proton, the stronger the base.
So, you can see why the more alkyl groups attached to the nitrogen, the stronger the basicity of the amine. It’s all about that electron density, folks!
Here’s a deeper dive into the electron-donating effect:
The alkyl group in ethyl amine has a positive inductive effect. This means it pushes electron density towards the nitrogen atom. This effect arises from the sigma bonds between the carbon atoms in the alkyl group and the nitrogen atom. The sigma bonds have a higher electron density closer to the more electronegative atom (nitrogen).
In contrast, ammonia has no alkyl group, so it doesn’t have this positive inductive effect. The nitrogen in ammonia has less electron density compared to ethyl amine, which makes it less attracted to a proton. This results in ethyl amine being a stronger base than ammonia.
You can think of the alkyl group as a little electron-pushing machine. The more alkyl groups you have attached to the nitrogen, the more electron density it receives, making it a stronger base. This is why ethyl amine is a stronger base than ammonia, and why tertiary amines are generally the strongest bases among the amines.
Do alkyl groups increase the basicity of amines?
Let’s break this down further:
What is basicity? Basicity refers to a molecule’s ability to accept a proton (H+). A stronger base readily accepts protons.
Why do alkyl groups increase basicity? Alkyl groups are composed of carbon and hydrogen. They are electron-rich, meaning they have a higher electron density than the nitrogen atom. This extra electron density is pushed towards the nitrogen atom, increasing the negative charge on the nitrogen lone pair. This makes the nitrogen more willing to donate its lone pair to accept a proton, increasing the amine’s basicity.
An example: Compare ammonia (NH3) and methylamine (CH3NH2). Methylamine has a methyl group (CH3) attached to the nitrogen. This methyl group donates electrons to the nitrogen, making it more basic than ammonia.
What about steric effects? You mentioned steric effects. While alkyl groups generally increase basicity, steric hindrance can play a role, especially with larger alkyl groups. If the alkyl groups are bulky enough, they can hinder the approach of a proton to the nitrogen atom. This can decrease basicity. However, this effect is usually less significant than the electron-donating effect of the alkyl groups.
How do we quantify the base strength of amines?
We can quantify base strength using the pKa value. The pKa value is the negative logarithm of the Ka value, which is the acid dissociation constant. The pKa value provides a measure of the strength of an acid. The lower the pKa value, the stronger the acid. Since amines are bases, their pKa values reflect the strength of their conjugate acids. Therefore, a higher pKa value indicates a stronger base.
Let’s compare ammonia and methylamine again:
Ammonia (NH3): pKa of conjugate acid (NH4+) = 9.25
Methylamine (CH3NH2): pKa of conjugate acid (CH3NH3+) = 10.64
As you can see, methylamine has a higher pKa value, indicating that it is a stronger base than ammonia.
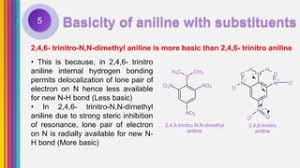
Why is p methyl aniline more basic than aniline?
p-Methylaniline is more basic than aniline because the methyl group is an electron-donating group. This means it pushes electron density towards the nitrogen atom in the amino group, making it more available to accept a proton. This increased electron density makes the nitrogen atom in p-methylaniline more likely to accept a proton, increasing its basicity.
Now, things get interesting when the methyl group is in the *ortho* position. Here, o-methylaniline is actually slightly less basic than aniline. This is due to the *steric effect* of the methyl group. The methyl group is bulky and can get in the way of the nitrogen atom’s ability to accept a proton.
Think of it this way: The methyl group in the *ortho* position acts like a shield, making it harder for a proton to get to the nitrogen. This steric hindrance reduces the basicity of o-methylaniline.
However, there’s more to it than just steric hindrance. The methyl group also has an *electronic effect*. The methyl group is electron-donating, but because it’s in the *ortho* position, it can’t donate as much electron density to the nitrogen atom. This is because the methyl group is too close to the nitrogen and creates some repulsion.
Essentially, the *ortho* methyl group has a more subtle influence than the *para* methyl group. It’s a combination of steric and electronic effects that ultimately lead to a decrease in basicity.
See more new information: linksofstrathaven.com
Why Methylamine Is More Basic Than Aniline: A Simple Explanation
Let’s get into the nitty-gritty of why methylamine is more basic than aniline. It all boils down to the electron-donating and electron-withdrawing effects of the groups attached to the nitrogen atom.
The Basics of Basicity
You know, basicity is all about how readily a molecule can accept a proton (H+). The more willing a molecule is to grab that proton, the more basic it is.
Now, let’s look at our two stars: methylamine (CH3NH2) and aniline (C6H5NH2). They both have a nitrogen atom with a lone pair of electrons that can snag a proton. But there’s a twist.
The Role of the Benzene Ring
In aniline, that nitrogen is directly connected to a benzene ring. This ring is a bit of a tricky character. It’s got these delocalized electrons that are constantly moving around.
This delocalization means the electrons in the ring are more stable and less available to donate to the nitrogen. This, in turn, makes the nitrogen in aniline less likely to grab a proton, making it less basic than methylamine.
Methylamine’s Advantage
Methylamine is different. The nitrogen is linked to a methyl group (CH3), which is a simple, straightforward group. The methyl group is an electron-donating group. This means it pushes electrons towards the nitrogen, making the lone pair on the nitrogen more available for grabbing that proton. Methylamine becomes a more willing proton acceptor and thus more basic.
Think of it This Way
Imagine the nitrogen is like a hungry person. The lone pair is like the food it wants to eat (a proton). In aniline, the benzene ring is a picky eater and keeps the food for itself, making the nitrogen hungry and less eager to grab food. But in methylamine, the methyl group is generous and shares its food with the nitrogen, making the nitrogen happier and more likely to grab food (that proton).
The Bottom Line
The benzene ring in aniline pulls electron density away from the nitrogen, making it less basic, while the methyl group in methylamine pushes electron density towards the nitrogen, making it more basic.
A Quick Recap
* Methylamine is more basic than aniline due to the electron-donating nature of the methyl group.
* Aniline is less basic due to the electron-withdrawing nature of the benzene ring.
FAQs
1. What is basicity, and why is it important?
Basicity is a measure of how readily a molecule can accept a proton. It’s crucial in chemistry because it determines how molecules interact with acids and other compounds.
2. How do you determine the basicity of a molecule?
You can determine the basicity of a molecule by looking at the structure and the electron-donating or -withdrawing groups attached to the atom that’s accepting the proton.
3. Why is the benzene ring electron-withdrawing?
The benzene ring is electron-withdrawing because of the delocalization of electrons in the ring. This delocalization makes the electrons more stable and less likely to be donated to other atoms.
4. What are some examples of electron-donating groups?
Some examples of electron-donating groups include alkyl groups like methyl (CH3), ethyl (CH2CH3), and propyl (CH2CH2CH3).
5. Why is basicity important in organic chemistry?
Basicity is crucial in organic chemistry for understanding reactions, predicting product formation, and controlling reaction pathways.
I hope this article shed some light on why methylamine is more basic than aniline. If you have any other questions, feel free to ask!
24.4: Basicity of Arylamines – Chemistry LibreTexts
Basicity of Aniline. Aniline is substantially less basic than methylamine, as is evident by looking at the pK a values for their respective ammonium conjugate acids (remember that the lower the pKa of the conjugate acid, the weaker the base). Chemistry LibreTexts
23.1: Relative Basicity of Amines and Other Compounds
Basicity of aniline. Aniline is substantially less basic than methylamine, as is evident by looking at the pK a values for their respective ammonium conjugate acids (remember Chemistry LibreTexts
21.4: Acidity and Basicity of Amines – Chemistry LibreTexts
In the case of para-methoxyaniline, the lone pair on the methoxy group donates electron density to the aromatic system, and a resonance contributor can be Chemistry LibreTexts
Basicity of Amines – Chemistry Steps
For example, ethyl amine is more basic than ammonia because the electron donating effect of the alkyl group increases the electron density on the nitrogen and thus Chemistry Steps
24.4 Basicity of Arylamines – Organic Chemistry | OpenStax
Substituted arylamines can be either more basic or less basic than aniline, depending on the substituent. Electron-donating substituents, such as –CH 3, –NH 2, and –OCH 3, OpenStax
CHAPTER 21: AMINES – University of Texas at Austin
The common name for this very simple amine is methylamine (no separators between methyl and amine). The secondary amine which has one methyl group and one ethyl group attached to nitrogen is named N UT Austin Chemistry & Biochemistry
amines as bases – chemguide
AMINES AS BASES. This page looks at the reactions of amines as bases. Their basic properties include the reactions with dilute acids, water and copper (II) ions. It only deals chemguide
Explaining the strength of organic bases – chemguide
Why are aliphatic primary amines stronger bases than ammonia? Methylamine. Methylamine has the structure: The only difference between this and ammonia is the presence of the CH 3 group in the chemguide
organic chemistry – Why is aniline less basic than methylamine …
Therefore, the HOMO of aniline has a poorer energy match with the H 1s LUMO than the HOMO of methylamine, and the resonance energy stabilizing the Chemistry Stack Exchange
Why Aniline Is Less Basic Than Methyl Amine ?(Amines | Class12 )
Account For The Following : Aniline Is Weaker Base Than Methylamine…
Why Is Ethyl Amine More Basic Than Aniline?
Why Aniline Is Less Basic Than Methylamine? | Organic Chemistry Reason | Notes
#Darkomega #Cbsc #Bseb #Question Aniline Is Less Basic Than Methylamine
(L-17) Which Is More Basic Aniline V/S Ammonia | Ncert Q\U0026A | Jee Neet | By Arvind Arora
Why Is Ethyl Amine A Stronger Base Than Ammonia?
Link to this article: why methylamine is more basic than aniline.
See more articles in the same category here: https://linksofstrathaven.com/how
