Are H2O and co2 organic?
The key to understanding organic compounds lies in their composition. To be classified as organic, a compound must contain carbon and hydrogen. Carbon dioxide (CO2) contains carbon but lacks hydrogen. Similarly, water (H2O) contains hydrogen but not carbon.
Since both CO2 and H2O lack either carbon or hydrogen, they fall outside the definition of organic compounds and are considered inorganic.
Delving Deeper into Organic Compounds
You might wonder, why is carbon so special in the world of organic compounds? The answer lies in carbon’s unique ability to form long chains and complex structures with other carbon atoms and other elements, particularly hydrogen. This versatility allows for a vast array of different organic compounds with diverse properties and functions.
Think of it like building with LEGO blocks. Carbon is like the fundamental LEGO brick, and hydrogen is another essential piece. By combining these and other elements, you can create a seemingly endless variety of structures, just like nature does with organic compounds. From simple molecules like methane (CH4) to complex biomolecules like proteins and DNA, carbon plays a central role in shaping the world around us.
In contrast, inorganic compounds like carbon dioxide (CO2) and water (H2O) are generally simpler in structure and often play vital roles in maintaining life but don’t possess the same complexity and diversity as organic compounds.
Why is water not considered an organic molecule?
Think of it this way: Carbon is like the building block for organic molecules. It has the unique ability to bond with itself and other elements in many different ways, forming long chains and complex structures. These structures are the foundation for the huge variety of molecules that make up living things, like proteins, carbohydrates, and fats.
Water, on the other hand, is a simple inorganic molecule that plays a vital role in life. It’s essential for many biological processes, like transporting nutrients and regulating temperature. But because it doesn’t have carbon, it doesn’t fit the definition of an organic molecule.
Is water organic, inorganic, or neither?
Think of it this way: Water is a building block for life, but it’s not life itself. It’s like the bricks in a house. Bricks are essential for building a house, but they aren’t a house on their own.
Similarly, water is fundamental to the existence of life, but it’s not considered a living organism. It’s more accurate to say that water is a component of life, not a form of life itself.
Is h2o water an organic compound?
Water is a compound made up of two hydrogen atoms and one oxygen atom, held together by covalent bonds. The key here is that organic compounds always contain carbon atoms. Since water doesn’t have any carbon atoms, it falls into the inorganic category.
Think of it this way: organic compounds are often associated with life, like the carbohydrates, proteins, and fats that make up our bodies. These compounds are complex and usually contain carbon as their backbone. Inorganic compounds, on the other hand, are often simpler and found in the non-living world. Water, for instance, is essential for life, but it’s not a building block of life itself.
Inorganic compounds can be found in various forms. Minerals like salt and quartz are inorganic, as are many gases like oxygen and nitrogen. They are essential for maintaining the balance of our planet, but they don’t have the intricate carbon-based structures that define organic compounds.
So, while water is crucial for all life, it’s not considered an organic compound because it lacks the key ingredient – carbon.
Is pure water organic or inorganic?
Let’s dive a little deeper into why water is considered inorganic. The definition of an organic compound is a compound that contains carbon, and often, hydrogen. Organic chemistry is the study of these compounds, and it’s a massive field encompassing everything from plastics to proteins!
However, water, though essential for life, doesn’t have that crucial carbon atom. This is why it’s classified as inorganic. It’s worth noting that water can participate in organic reactions, helping to form and break down organic molecules. But this doesn’t change its fundamental inorganic nature.
Why is CO2 not organic?
The key is the hydrogen. Organic molecules must contain carbon bonded to hydrogen. While CO2 has carbon, it doesn’t have hydrogen attached to it. Think of it like this: Organic molecules are like a team, and carbon and hydrogen are the core players. CO2 is missing a key player, the hydrogen, so it can’t be part of the organic team.
To put it simply, organic compounds are built from a framework of carbon and hydrogen. This framework is the foundation for a huge variety of molecules, like sugars, fats, and proteins, that make up the building blocks of life. CO2 doesn’t have that foundation. It’s like a building without walls and a roof – it can’t be considered a complete structure.
Let’s dive deeper into why hydrogen is so important in the world of organic chemistry. The carbon-hydrogen bond is unique. It’s the foundation for all organic compounds, and it’s what allows these compounds to be so diverse and complex. The hydrogen atoms are able to form different types of bonds, including single, double, and triple bonds, allowing for a wide range of shapes and structures in organic molecules. This diversity is what makes organic chemistry so interesting and important.
CO2, on the other hand, is a much simpler molecule. It has two oxygen atoms bonded to a single carbon atom. This simple structure makes it a stable and inert molecule. It’s not a building block of life, but it plays an important role in the environment, like helping to regulate Earth’s temperature.
So, the next time you see the term organic, remember that it’s all about the presence of carbon bonded to hydrogen. CO2 may have carbon, but it’s missing the vital hydrogen to be considered organic.
How to know if organic or inorganic?
Let’s break that down further. You know how everything is made up of tiny building blocks called atoms? Well, carbon is one of those really important atoms. It’s a bit of a social butterfly and loves to bond with other atoms, especially hydrogen. This bonding creates the framework for a huge variety of organic molecules, from the sugars in your coffee to the proteins in your muscles.
Think of it like this: organic compounds are like the “life” molecules. They’re the building blocks of everything from plants and animals to the food we eat. Inorganic compounds, while super important, don’t usually have the same intricate structures and are more likely to be found in minerals, rocks, and even the air we breathe.
So, next time you’re trying to figure out if something is organic or inorganic, just ask yourself: Does it contain carbon, especially bonded to hydrogen? If yes, it’s likely organic. If not, it’s probably inorganic. Simple as that!
Is hydrogen organic or inorganic?
Organic compounds are typically built around a framework of carbon atoms. They often include hydrogen, oxygen, and nitrogen, but they can also have other elements like phosphorus, sulfur, silicon, and halogens. The presence of carbon is the defining feature of an organic compound.
Now, hydrogen is a pretty versatile element and shows up in both organic and inorganic compounds. Think of water (H₂O) – that’s inorganic! But, hydrogen is also a key player in many organic compounds like methane (CH₄) and glucose (C₆H₁₂O₆).
So, the key takeaway is that hydrogen itself isn’t inherently organic or inorganic. It’s the company it keeps that determines its classification. If hydrogen is hanging out with carbon, it’s likely part of an organic compound. But if it’s bonded to elements like oxygen or chlorine, it’s likely part of an inorganic compound.
Let’s break down why carbon plays such a starring role in organic chemistry. It’s a master of bonding! Carbon can form strong, stable bonds with itself and other elements, creating a vast array of complex molecules. These carbon-based structures can be chains, rings, or even three-dimensional networks, which allows for an incredible diversity of organic compounds.
From the carbohydrates that fuel our bodies to the proteins that build our cells, from the DNA that carries our genetic code to the plastics that make up so many everyday objects, organic chemistry is all about the incredible versatility of carbon and its ability to form these complex structures with the help of hydrogen and other elements.
Why can’t water be organic?
Similarly, salt (sodium chloride) is inorganic because it lacks carbon. Clay is also inorganic, made up of various minerals. These compounds are essential for the earth and its processes but are not considered organic.
To delve deeper into the heart of this topic, let’s break down why carbon is so crucial for organic compounds:
Carbon’s Bonding Power: Carbon has an exceptional ability to form strong bonds with other carbon atoms and various other elements, including hydrogen, oxygen, nitrogen, and phosphorus. These bonds create a diverse range of complex molecules, like proteins, carbohydrates, lipids, and nucleic acids, which are the building blocks of life.
Carbon’s Versatility: Carbon can form single, double, and triple bonds, allowing for a vast array of possible molecular structures. This versatility makes carbon the ideal element for creating the complex molecules necessary for life.
Carbon’s Stability: Carbon bonds are relatively stable, making them ideal for forming the structures that support and sustain life. These stable bonds ensure the molecules don’t easily fall apart.
So, while water is essential for life and exists in abundance, it simply doesn’t contain the key ingredient—carbon—that defines organic compounds. This makes it a crucial inorganic compound that plays a vital role in supporting life without being organic itself.
See more here: Are H2O And Co2 Organic? | Is H2O Organic Or Inorganic
Is water an inorganic compound?
Water is indeed an inorganic compound with the chemical formula H2O. It’s a transparent, tasteless, odorless, and nearly colorless substance. You might be surprised to learn that water is the main component of Earth’s hydrosphere and the fluids of all living things. It acts as a solvent in these living organisms.
But what exactly makes waterinorganic? The key is that water does not contain carbon-hydrogen bonds, which are the hallmark of organic compounds. It’s made up of two hydrogen atoms and one oxygen atom, forming a simple but vital molecule. While water doesn’t contain carbon-hydrogen bonds, it’s crucial to life as we know it. It plays a vital role in many biological processes, from photosynthesis in plants to the transportation of nutrients and waste in animals.
In essence, water’s lack of carbon-hydrogen bonds classifies it as inorganic. But don’t let that fool you! Water is far from simple, as it’s essential for the existence and function of all life on Earth.
Is H2O inorganic?
Inorganic compounds, unlike organic compounds, are generally simple molecules that lack carbon atoms. They play crucial roles in many biological processes, making them vital for life. Water, being an inorganic compound, is an excellent example of this.
Think of it this way: while you need to eat organic compounds like carbohydrates, fats, and proteins to provide your body with energy and building blocks for growth, your body also needs inorganic compounds like water to regulate temperature, transport nutrients, and facilitate chemical reactions. It’s a team effort!
Let’s dive a little deeper into why H2O is inorganic. The definition of an inorganic compound centers around the presence or absence of carbon. While organic compounds are defined by the presence of carbon, inorganic compounds lack this key element. Water, with its simple molecular structure consisting of two hydrogen atoms and one oxygen atom, fits neatly into the category of inorganic compounds.
It’s important to note that there are exceptions to the rule. Some inorganic compounds contain carbon, such as carbon dioxide (CO2) and carbonates like calcium carbonate (CaCO3). However, these compounds are typically considered inorganic because they don’t have the complex structures and properties associated with organic molecules.
So, to sum it up, while we might not think about it often, water is an essential inorganic compound that plays a vital role in our lives.
Is water organic or inorganic?
From a chemist’s perspective, water is inorganic. The chemical formula for water is H2O, meaning it consists of two hydrogen atoms bonded to one oxygen atom. Since it lacks carbon, a key ingredient in all organic molecules, it falls under the inorganic category.
However, the answer gets a bit more nuanced when we consider the perspective of a farmer. Farmers might consider water natural and even organic if it’s sourced from a natural spring or hasn’t been treated with synthetic additives. This perspective emphasizes the origin and purity of the water rather than its chemical composition.
Now, let’s break down the organic versus inorganic classification a bit further. Organic molecules are essentially the building blocks of life. They contain carbon bonded to hydrogen atoms, and often other elements like oxygen, nitrogen, sulfur, and phosphorus. They form complex structures like carbohydrates, proteins, lipids, and nucleic acids which are essential for life processes.
Inorganic molecules, on the other hand, are typically simpler compounds that don’t contain carbon bonded to hydrogen. These include substances like water, salts, minerals, and gases. While they might not be directly involved in life processes like organic molecules, they play crucial roles in maintaining life. For example, water is essential for hydration and various biological reactions, and minerals like calcium are critical for bone health.
So, when it comes to water, both the chemist and the farmer have valid points. Water is inorganic based on its chemical composition, but its source and purity can be considered organic depending on how it’s used and obtained. Understanding both perspectives allows us to appreciate the multifaceted nature of water and its role in our lives.
Is H2O a liquid or a gas?
Under normal conditions, H2O exists as a liquid. You know it as the stuff we drink, swim in, and use to water our plants. But did you know that water can also exist as a solid (ice) and a gas (water vapor)?
Here’s the thing. The temperature and pressure around us play a big role in how H2O behaves. On Earth, we’re lucky enough to live in an environment where water can be all three states – solid, liquid, and gas. Think about it: ice cubes in your drink, the water in your glass, and the clouds in the sky.
But H2O is a real chameleon! It can switch between these states based on the conditions around it. That’s why we see rain, snow, and fog. Rain is simply water vapor in the atmosphere that condenses into liquid water. Snow happens when water vapor freezes directly into ice crystals, and fog is just water vapor that has condensed into tiny water droplets.
So, to answer your question: H2O is a liquid under normal conditions, but it can also exist as a solid (ice) and a gas (water vapor). The form it takes depends on the temperature and pressure of its surroundings.
See more new information: linksofstrathaven.com
Is H2O Organic Or Inorganic: A Simple Explanation
Alright, let’s dive into the world of chemistry and figure out if H2O, which is just a fancy way of saying water, is organic or inorganic.
You might be thinking, “Wait, water? That’s everywhere! How can it be one or the other?” And you’d be right, water is incredibly important. But, when it comes to organic chemistry, it’s all about the carbon.
The Key to Organic Chemistry: Carbon
Think of carbon as the building block of life. It can form strong bonds with other atoms, like hydrogen, oxygen, and nitrogen. These bonds allow for complex molecules to form, and organic molecules are all about those complex structures.
So, if carbon is the star of the show in organic chemistry, where does water fit in? Well, let’s break it down:
H2O is made up of hydrogen and oxygen.
* It doesn’t contain carbon.
* That means water doesn’t meet the criteria for being an organic molecule.
The Verdict: H2O Is Inorganic
Therefore, H2O is considered inorganic.
You might be thinking, “But water is essential for life! How can it be inorganic?” It’s a great question, and it gets to the heart of how we categorize things in chemistry.
While water is super important for living things, its composition doesn’t include carbon, which is the defining feature of organic molecules.
Putting it All Together
So, the answer is clear: H2O is inorganic. We can summarize it like this:
Organic molecules contain carbon.
Water (H2O) doesn’t contain carbon.
* Therefore, water is considered inorganic.
Exploring Organic and Inorganic Compounds
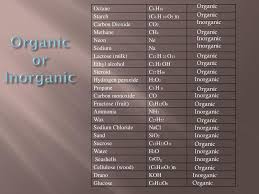
Let’s explore some more examples of organic and inorganic compounds:
Organic Compounds:
Glucose (C6H12O6): The primary source of energy for living things. Contains carbon.
Methane (CH4): A simple hydrocarbon found in natural gas. Contains carbon.
Proteins: Made up of chains of amino acids, which contain carbon.
DNA: Carries genetic information and is composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus.
Inorganic Compounds:
Carbon dioxide (CO2): A greenhouse gas. Contains carbon, but it’s not considered organic because it’s a simple molecule.
Sodium chloride (NaCl): Table salt. Doesn’t contain carbon.
Iron oxide (Fe2O3): Rust. Doesn’t contain carbon.
Water (H2O): The universal solvent. Doesn’t contain carbon.
FAQs
Q: What are some common uses of inorganic compounds?
A: Inorganic compounds have a wide range of uses. Think about things like:
Building materials: Concrete, glass, and steel are all examples of inorganic compounds.
Industrial applications: Inorganic compounds are used in fertilizers, pesticides, and manufacturing processes.
Medicine: Many medications are inorganic compounds.
Cleaning products: Inorganic compounds are often used in cleaning solutions.
Q: Can something be both organic and inorganic?
A: It’s a tricky question! Some substances can have both organic and inorganic components, but the core definition of “organic” still revolves around the presence of carbon.
Q: How can I tell if a compound is organic or inorganic?
A: Look for the presence of carbon in the chemical formula. If it contains carbon, it’s likely organic. If it doesn’t contain carbon, it’s likely inorganic.
Q: Is it important to know the difference between organic and inorganic compounds?
A: Absolutely! Understanding the difference between organic and inorganic compounds is crucial for several reasons:
Chemistry: It helps us understand the fundamental properties and reactions of different substances.
Biology: It’s important for understanding how living things function and interact with their environment.
Environmental science: It helps us understand how different chemicals affect the environment.
Q: Can something be organic but not contain carbon?
A: While carbon is the key element in organic chemistry, there are a few exceptions. Some compounds, like carbon dioxide (CO2), technically contain carbon but aren’t considered organic due to their simple structure and lack of complex carbon chains.
Q: What about compounds with carbon that aren’t considered organic?
A: There are a few examples of compounds that contain carbon but aren’t considered organic. These include simple carbon-containing compounds like carbon dioxide (CO2) and carbon monoxide (CO), which don’t have the complex structures characteristic of organic molecules.
Wrapping It Up
Now you have a solid understanding of why water (H2O) is classified as inorganic. Remember, organic means containing carbon, and water doesn’t have that crucial element.
Is Water (H2O) an Organic or Inorganic Molecule? – YouTube
Although water is inorganic, it is made up of nonmetals, just like organic compounds. Therefore H2O has covalent bonds where the electrons between atoms are shared. Organic compounds also have… YouTube
Is Water Organic? – The Green Chemist
The answer to the question depends on the perspective of the person answering. A chemist would say no, a farmer or an organic cosmetic certification body would say no or natural, and a natural The Green Chemist
Difference Between Organic and Inorganic – Science
Organic compounds contain carbon, usually bonded to hydrogen. Inorganic compounds either don’t contain carbon or else it is bonded to oxygen, nitrogen, or a metal. The terms “organic” and Science Notes and Projects
Is Water a Compound or an Element? – ThoughtCo
The chemical formula for water is H 2 O, which means each molecule of water consists of one oxygen atom chemically bonded to two hydrogen atoms. Thus, water is a compound. It’s also a molecule, which ThoughtCo
The Difference Between Organic and Inorganic
Organic compounds contain carbon and hydrogen, while inorganic compounds do not. Learn the difference between organic and inorganic, plus examples of each type, such as H2O and CO2. ThoughtCo
3.6: Names and Formulas of Inorganic Compounds
This and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic Chemistry LibreTexts
Organic vs. Inorganic Molecules — Definition
Some incredibly important inorganic molecules and compounds include the oxygen you breathe (O2) and the water you drink (H2O). Here’s a chart with some important organic and inorganic molecules: Image source: By Expii
Is Water (H2O) An Organic Or Inorganic Molecule?
Is H2O(Water) Organic Or Inorganic Compound?||Is Water An Organic Molecule?
Difference Between Organic And Inorganic Compounds
Why Is H2O(Water) An Inorganic Compound?
Difference Between Organic And Inorganic Compounds
Organic Vs Inorganic
Water And Inorganic Minerals
Link to this article: is h2o organic or inorganic.
See more articles in the same category here: https://linksofstrathaven.com/how
