What happens when mercury (II) oxide is heated?
Mercury(II) oxide, a bright red solid, undergoes a transformation when heated. This process, known as decomposition, breaks down the compound into its simpler components: mercury (a silvery liquid metal) and oxygen gas. It’s like taking a puzzle apart, separating the pieces into their original forms.
Think of it this way: mercury(II) oxide is a stable compound at room temperature. But when you apply heat, you’re essentially providing the energy needed to break the chemical bonds holding the mercury and oxygen atoms together. This energy causes the mercury(II) oxide to decompose, releasing the mercury and oxygen as separate substances.
Here’s a closer look at the chemical equation for this decomposition reaction:
2HgO → 2Hg + O₂
This equation tells us that two molecules of mercury(II) oxide (HgO) decompose to produce two atoms of mercury (Hg) and one molecule of oxygen gas (O₂).
This reaction is a classic example of a decomposition reaction, where a single compound breaks down into two or more simpler substances. Decomposition reactions often require energy input, typically in the form of heat, light, or electricity, to break the chemical bonds.
Now, let’s imagine this reaction happening in a test tube. You’d see the red mercury(II) oxide gradually turn black as it decomposes. The black color is a telltale sign of the formation of liquid mercury. You might also notice tiny droplets of mercury forming on the cooler parts of the test tube. And, of course, the release of oxygen gas is another indicator of the decomposition process.
In a nutshell, heating mercury(II) oxide is a fascinating chemical transformation that illustrates the principles of decomposition reactions. It’s a beautiful example of how energy can be used to break down compounds and release their constituent elements.
What happens when mercuric oxide is heated with equation?
When you heat mercuric oxide (HgO), it breaks down into its component elements: mercury (Hg) and oxygen (O2). This process is called decomposition.
The chemical equation that represents this reaction is:
2HgO + 180 kJ → 2Hg + O2
This equation tells us a few important things:
2 moles of mercuric oxide (HgO) react.
180 kJ of heat energy is absorbed (indicated by the plus sign). This means the reaction is endothermic.
2 moles of mercury (Hg) are produced.
1 mole of oxygen (O2) is produced.
The Heat of Formation
The heat of formation of a compound is the change in enthalpy that occurs when one mole of the compound is formed from its elements in their standard states. In this case, the heat of formation of mercuric oxide is the amount of heat released or absorbed when one mole of mercuric oxide is formed from its elements, mercury and oxygen, in their standard states.
The value provided in the original text (5.66 kJ/mole) is incorrect. The heat of formation of mercuric oxide is actually -90.83 kJ/mole. This means that the formation of mercuric oxide is an exothermic reaction, releasing heat.
The Importance of the Decomposition of Mercuric Oxide
The decomposition of mercuric oxide is a classic example of a chemical reaction that demonstrates the principles of conservation of mass and energy. It is also an important reaction in the history of chemistry.
* Joseph Priestley, a British chemist, is credited with discovering oxygen in 1774 through the decomposition of mercuric oxide.
Antoine Lavoisier, a French chemist, later used the decomposition of mercuric oxide to help develop the law of conservation of mass.
How the Decomposition Happens
When you heat mercuric oxide, the heat energy causes the Hg-O bonds to break. The mercury atoms are then free to combine to form mercury liquid, while the oxygen atoms combine to form oxygen gas.
A Closer Look at the Reaction
Mercuric oxide is a red, crystalline solid.
Mercury is a silvery, liquid metal.
Oxygen is a colorless, odorless gas.
The decomposition of mercuric oxide is a reversible reaction, meaning it can proceed in both directions. However, at room temperature, the equilibrium lies far to the right, meaning that the mercuric oxide will decompose almost completely into mercury and oxygen.
Safety Precautions
It is important to note that mercury is a toxic substance. It is important to handle mercuric oxide and mercury with care. Avoid inhaling mercury vapors or skin contact. If you are working with mercuric oxide, be sure to wear appropriate safety gear, such as gloves and a mask.
I hope this explanation has helped you understand what happens when you heat mercuric oxide!
When solid mercury (II) oxide is heated?
When you heat solid mercury (II) oxide, it undergoes a fascinating transformation, breaking down into liquid mercury and oxygen gas. This process, called decomposition, is a chemical reaction where a single compound breaks down into two or more simpler substances.
Let’s delve deeper into this reaction:
Imagine you have a sample of mercury (II) oxide – it’s a red, powdery substance. When you heat it, you’re providing energy to the mercury (II) oxide molecules. This energy causes the mercury (II) oxide molecules to vibrate faster and break apart. The bonds holding the mercury and oxygen atoms together weaken and eventually break.
The broken bonds release the mercury and oxygen atoms as separate substances. The mercury, now in its liquid state, collects at the bottom of the container, while the oxygen gas escapes into the air.
This decomposition reaction is represented by the following chemical equation:
2HgO(s) → 2Hg(l) + O2(g)
Where:
HgO represents mercury (II) oxide, a solid (s)
Hg represents mercury, a liquid (l)
O2 represents oxygen, a gas (g)
This simple reaction has some interesting applications. It’s historically significant as it was one of the first methods used to produce oxygen gas. It also demonstrates the principle of conservation of mass – the total mass of the reactants (mercury (II) oxide) equals the total mass of the products (mercury and oxygen).
What is the formula for HgO?
HgO is a fascinating compound with a rich history. It’s been used for centuries in various applications, from pigments and batteries to pharmaceuticals and even explosives. Let’s delve deeper into its unique properties:
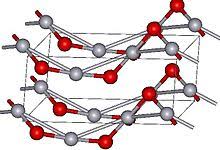
Structure: HgO exists in two main forms: a red form, which is more stable, and a yellow form. These forms differ slightly in their crystal structures. The red form has a cinnabar structure, meaning it forms a layered lattice with each mercury atom bonded to two oxygen atoms. The yellow form has a calomel structure, a linear arrangement of mercury and oxygen atoms. Interestingly, the yellow form can be converted to the red form by heating it.
Reactions:HgO is a valuable reagent in chemical reactions. It’s often used as a source of mercury for chemical synthesis. For instance, when heated, it decomposes to form elemental mercury and oxygen gas. This decomposition reaction is also used in the production of oxygen for breathing apparatuses and certain chemical processes.
Applications: HgO has a wide range of applications. It’s used as a pigment in paints, inks, and ceramics, where its bright red color adds a striking visual impact. In batteries, HgO serves as the cathode material, contributing to the long shelf life of mercury batteries. Furthermore, HgO is used in pharmaceuticals to create specific ointments and creams.
HgO is a versatile compound with unique properties that make it a crucial component in various industries. Understanding its structure, reactions, and applications is crucial for appreciating its significance in chemistry, medicine, and technology.
What is the balanced formula for mercury II oxide?
Let’s break down why this is the case:
Mercury (II) oxide is an inorganic compound that forms when mercury is exposed to oxygen. The Roman numeral II indicates the oxidation state of mercury in the compound, which is +2. This means that each mercury atom loses two electrons, while each oxygen atom gains two electrons to form the HgO molecule.
The balanced equation for the decomposition of mercury (II) oxide is:
2HgO + heat –> 2Hg + O2
This equation indicates that when mercury (II) oxide is heated, it decomposes into its constituent elements: liquid mercury and oxygen gas. The equation is balanced because it shows an equal number of atoms of each element on both sides of the equation.
The balanced equation shows that two moles of HgO produce two moles of Hg and one mole of O2. This is important because it tells us the stoichiometry of the reaction, or the relative amounts of reactants and products involved in the reaction.
The decomposition of mercury (II) oxide is an important reaction in the production of mercury. It is also used in the manufacture of batteries, pigments, and fungicides.
Here’s a deeper look at the decomposition process:
Heat is the key to breaking the bonds in HgO. When heat is applied, the mercury atoms gain enough energy to overcome the attraction between them and the oxygen atoms, leading to the formation of liquid mercury and oxygen gas.
The decomposition of mercury (II) oxide is a reversible reaction, meaning it can proceed in both directions. At low temperatures, the reaction favors the formation of HgO. However, at high temperatures, the reaction favors the decomposition of HgO into its constituent elements.
In essence, the balanced formula for mercury (II) oxide, HgO, plays a crucial role in understanding the decomposition process and its applications.
Which type of chemical reaction is the equation 2 HgO -> 2 Hg O2?
Let’s break down the concept of thermal decomposition a little further. Thermal decomposition reactions occur when a compound is unstable at high temperatures and requires an input of energy, typically in the form of heat, to initiate the reaction. This energy input overcomes the bonds holding the compound together, causing it to break apart into simpler substances.
Thermal decomposition is a common type of chemical reaction with various applications. For example, it is used in the production of certain metals, such as magnesium and aluminum, and in the decomposition of carbonates to produce carbon dioxide.
In the case of the thermal decomposition of mercury(II) oxide, the reaction is characterized by the following:
Reactant:Mercury(II) oxide (HgO)
Products:Liquid mercury (Hg) and oxygen gas (O2)
Energy Input: Heat is required to break the bonds in mercury(II) oxide.
Reaction Conditions: The reaction typically occurs at high temperatures.
It’s important to remember that thermal decomposition reactions are not always straightforward. The products formed and the conditions required for the reaction can vary depending on the compound being decomposed. However, understanding the fundamental concept of thermal decomposition provides a basis for understanding various chemical reactions and their applications.
What is the heating effect of HgO?
This decomposition reaction is a key aspect of the heating effect of HgO. When you apply heat to HgO, you’re essentially providing the energy needed to break the bonds holding the mercury and oxygen atoms together in the HgO molecule. This leads to the formation of liquid mercury and gaseous oxygen.
Let’s break down the process:
Heat Energy Input: The initial energy input from the heat source causes the HgO molecules to vibrate more rapidly.
Bond Breaking: As the vibrations intensify, the bonds between the mercury and oxygen atoms within the HgO molecule weaken and eventually break.
Product Formation: This bond breaking results in the formation of free mercury atoms and oxygen atoms. The mercury atoms then condense into liquid mercury, while the oxygen atoms combine to form diatomic oxygen gas (O2).
This decomposition process is endothermic, meaning it absorbs heat from the surroundings. You can think of it like a sponge soaking up water. The HgO molecule absorbs heat energy to break apart.
It’s important to note that the decomposition of HgO occurs at a specific temperature. The exact temperature will depend on factors such as the purity of the HgO and the pressure of the surrounding environment. However, generally speaking, the decomposition process starts at around 500°C (932°F).
What happens when oxide is heated?
Mercury(II) oxide (HgO) is a special case because it decomposes at normal temperatures. When you heat it, it forms liquid mercury and oxygen gas. Other metal oxides require much higher temperatures to decompose.
Think of it like baking a cake. When you heat the cake batter, it breaks down into the ingredients you used – flour, sugar, eggs, etc. – and forms a delicious, fluffy cake! In a similar way, heating a metal oxide breaks it down into its component parts – the metal and oxygen.
Let’s dive deeper into the process of decomposition. When you apply heat to a metal oxide, you provide the energy needed to break the chemical bonds holding the metal and oxygen atoms together. The metal atoms are then free to move around and form a solid metal. The oxygen atoms combine to form oxygen gas, which is released into the atmosphere.
The temperature at which a metal oxide decomposes depends on the strength of the chemical bond between the metal and oxygen atoms. Some metal oxides, like mercury(II) oxide, have weak bonds and decompose easily. Others, like iron(III) oxide, have strong bonds and require much higher temperatures to break down.
Here are some examples of metal oxides and their decomposition temperatures:
Mercury(II) oxide (HgO): Decomposes at about 350°C
Iron(III) oxide (Fe2O3): Decomposes at about 1535°C
Copper(II) oxide (CuO): Decomposes at about 1026°C
Zinc oxide (ZnO): Decomposes at about 1975°C
Decomposition of Metal Oxides: The Bottom Line
Heating metal oxides breaks them down into their basic components: metal and oxygen. This decomposition temperature varies depending on the specific metal oxide. It is important to note that the decomposition process is irreversible; once the metal oxide has decomposed, it cannot be reformed simply by cooling it.
See more here: What Is The Balanced Equation For Hgo → Hg O2? | Mercury Ii Oxide Heated Formula
What temperature is mercuric oxide?
Mercuric oxide, also known as mercury oxide, has the chemical formula HgO. It’s a cool compound with a vibrant red or orange hue. At room temperature and pressure, it’s a solid. So, what about its temperature?
Well, mercuric oxide is a solid at room temperature, which is generally around 25 °C (77 °F). Now, the melting point of mercuric oxide is 500 °C (932 °F). This means it will melt and become liquid at this temperature. It’s important to remember that this is the melting point; mercuric oxide will remain solid below this temperature.
However, keep in mind that mercuric oxide can decompose into mercury and oxygen when heated to a high temperature. This decomposition process happens at a temperature of about 300 °C (572 °F). This means mercuric oxide won’t exist as a solid above this temperature; it will transform into mercury and oxygen.
To summarize, mercuric oxide is a solid at room temperature. It melts at 500 °C (932 °F) and decomposes into mercury and oxygen at 300 °C (572 °F).
What is mercuric oxide?
Let’s delve a little deeper. Mercuric oxide is quite reactive with most acids. When it encounters an acid, it transforms into mercury salts, like a chameleon changing colors. This reaction is a fundamental process in the world of chemistry, allowing scientists to create a diverse range of mercury compounds for various applications.
See more new information: linksofstrathaven.com
Mercury Ii Oxide Heated Formula | What Happens When Mercury (Ii) Oxide Is Heated?
Let’s talk about mercury(II) oxide, a fascinating compound. You might know it as a red powder, and it plays a crucial role in a variety of chemical reactions. Today, we’re diving into the heat aspect of this compound – specifically, what happens when you heat it up. It’s like a chemical magic trick, so let’s get started!
The Magic of Heat: Breaking Down Mercury(II) Oxide
Heating mercury(II) oxide, also known as mercuric oxide, can trigger a decomposition reaction. This means the compound breaks down into simpler substances. Here’s the magic:
The Formula:
The reaction we’re discussing is represented by this formula:
HgO(s) → Hg(l) + ½O2(g)
Let’s break this down:
HgO(s): This is the solid mercury(II) oxide we’re starting with.
Hg(l): This is the liquid mercury that’s produced during the reaction.
½O2(g): This represents the oxygen gas that’s also released as a product. It’s important to note the “½” here. It means that only half a molecule of oxygen is produced for every molecule of mercury(II) oxide that decomposes.
The Process of Decomposition
When you heat mercury(II) oxide, the heat energy is absorbed by the compound’s molecules. This absorbed energy causes the bonds holding the mercury and oxygen atoms together to weaken and break.
Think of it like pulling apart a puzzle! The heat provides the energy needed to separate the puzzle pieces (mercury and oxygen) from each other.
Once the bonds break, the mercury atoms are free to move around more easily, which is why they form a liquid. The oxygen atoms also combine to form oxygen gas.
A Visual Example: The Red Powder’s Transformation
Imagine you have a small pile of red mercury(II) oxide powder. As you heat it up, the powder starts to change color, turning darker and eventually black. At the same time, you’ll see tiny droplets of liquid mercury forming. This liquid mercury will eventually evaporate and become gaseous, creating a silver-like vapor.
This transformation is fascinating because it showcases the decomposition process. The red powder is decomposing into liquid mercury and oxygen gas.
Safety First: The Importance of Caution
Remember, mercury is a very toxic element, so handle it with extreme care. It’s best to avoid this decomposition experiment at home and leave it to professionals in a lab setting.
Real-World Applications: More Than Just a Lab Experiment
The decomposition of mercury(II) oxide isn’t just a cool lab demo. It has real-world applications, like:
Mercury Extraction: The decomposition reaction is used in the extraction of mercury from its ores.
Production of Oxygen: While not a primary method, this reaction can be a source of pure oxygen, though it’s not the most efficient way to obtain it.
FAQs
Q: What happens if you don’t heat mercury(II) oxide?
A: If you don’t heat it, it will remain as a solid, and the reaction won’t occur.
Q: Can you reverse the decomposition reaction?
A: Yes, you can! By reacting liquid mercury with oxygen gas under the right conditions, you can re-form mercury(II) oxide.
Q: Is the decomposition of mercury(II) oxide an endothermic or exothermic reaction?
A: It’s an endothermic reaction because it requires heat energy to occur.
Q: What is the temperature required for the decomposition?
A: The decomposition of mercury(II) oxide starts at around 350 degrees Celsius.
Q: Is it safe to heat mercury(II) oxide in a closed container?
A: Absolutely not! The decomposition process produces oxygen gas, and if the container is sealed, the pressure could build up, leading to an explosion.
Q: What are some other examples of decomposition reactions?
A: Some other common decomposition reactions include:
* The decomposition of hydrogen peroxide into water and oxygen gas.
* The decomposition of calcium carbonate into calcium oxide and carbon dioxide.
Q: Can I use mercury(II) oxide in my garden?
A: No, mercury(II) oxide is highly toxic, so it’s not safe to use in a garden.
Q: Is mercury(II) oxide used in any products I use daily?
A: While it’s not a common ingredient in everyday products, mercury(II) oxide was once used in batteries, but its use has been discontinued due to safety concerns.
Q: Where can I learn more about mercury(II) oxide?
A: You can find a lot of information about mercury(II) oxide on websites like Wikipedia and PubChem.
Let me know if you have any other questions! I’m happy to help you unravel the fascinating world of chemistry.
Mercury(II) oxide – Simple English Wikipedia, the free
Its chemical formula is HgO. It has mercury and oxide ions. The mercury is in the +2 oxidation state . Properties. It is a red or yellow solid. It is very toxic and corrosive. It Wikipedia
Mercury(II) oxide – Sciencemadness Wiki
Mercury (II) oxide. Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Mercury (II) oxide, or Sciencemadness Dot Org
11.5: Decomposition Reactions – Chemistry LibreTexts
Mercury (II) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas. \[2 \ce{HgO} \left( s \right) \rightarrow 2 \ce{Hg} \left( l \right) + \ce{O_2} \left( g Chemistry LibreTexts
Mercury(II) oxide | HgO | ChemSpider
Mercury(II) oxide. Molecular Formula HgO; Average mass 216.589 Da; Monoisotopic mass 217.965515 Da; ChemSpider ID 28626 ChemSpider
Mercury(II) oxide – Wikiwand
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula HgO. It has a red or orange color. Mercury(II) oxide is a Wikiwand
5.4.2: Decomposition Reactions – Chemistry LibreTexts
Mercury (II) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas. \[2 \ce{HgO} \left( s \right) \rightarrow 2 \ce{Hg} \left( l \right) + Chemistry LibreTexts
Mercury(II) oxide | H2HgO | ChemSpider
Mercury (II) oxide. Molecular Formula HHgO. Average mass 218.605 Da. Monoisotopic mass 219.981171 Da. ChemSpider ID 29316612. ChemSpider
Mercury(II) oxide – Oxford Reference
Quick Reference. A yellow or red oxide of mercury, HgO. The red form is made by heating mercury in oxygen at 350°C; the yellow form, which differs from the Oxford Reference
Mercury – Compounds, Oxides, Salts | Britannica
Mercury does not combine with oxygen to produce mercury(II) oxide, HgO, at a useful rate until heated to the range of 300 to 350 °C (572 to 662 °F). At temperatures of Britannica
Decomposition Mercury (Ii) Oxide And Oxygen
Decomposition Of Mercury (Ii) Oxide : How To Balance
Make Mercury Metal (Decomposition Of Mercury (Ii) Oxide)
Mercury Ii Oxide Decomposition
Stoichiometry – Decomposition Of Mercury Ii Oxide Example
Decomposition Of Mercury(Ii) Oxide
How To Write The Formula For Mercury (Ii) Oxide
Link to this article: mercury ii oxide heated formula.
See more articles in the same category here: https://linksofstrathaven.com/how