Does hydride shift occur in Sn2?
1,2-Hydride shifts and 1,2-methyl shifts are common in SN1 reactions because they help create a more stable carbocation. Think of it like this: the molecule is trying to find the most comfortable arrangement, and shifting a hydrogen or methyl group can make the carbocation more stable.
However, SN2 reactions happen in one step, and there’s no intermediate carbocation. It’s like a direct transfer of a group from one molecule to another, without any rearranging. This means there’s no chance for a hydride shift to occur.
Here’s a more detailed explanation:
SN1 reactions involve a two-step mechanism. The first step is the formation of a carbocation, which is a positively charged carbon atom. This carbocation is highly reactive and can undergo rearrangements to become more stable. One way to achieve this stability is through a hydride shift. In a hydride shift, a hydrogen atom bonded to a carbon atom adjacent to the carbocation migrates to the carbocation, forming a new bond. This shift results in a more stable carbocation, typically a tertiary carbocation, because the positive charge is now distributed over a larger number of atoms.
SN2 reactions, on the other hand, proceed through a concerted mechanism, meaning that bond breaking and bond formation occur simultaneously. The nucleophile attacks the carbon atom bearing the leaving group from the backside, resulting in a one-step process with no intermediate carbocation formation. Therefore, there’s no opportunity for a hydride shift to occur in an SN2 reaction.
How many times can hydride shift occur?
Let’s break down why this happens. Carbocation stability is a key factor in determining the likelihood of a hydride shift. A hydride shift, which is the movement of a hydrogen atom and its bonding electrons, is a way to rearrange a molecule to create a more stable carbocation.
The more substituted a carbocation is, the more stable it is. This means a tertiary carbocation is more stable than a secondary carbocation, which is more stable than a primary carbocation. So, if a hydride shift can create a more substituted carbocation, it’s more likely to occur.
Think of it this way: the molecule is trying to find the most stable form, and hydride shifts are one way to achieve that. If a hydride shift can make the carbocation more stable, the molecule will likely take that path.
Imagine a carbocation like a wobbly chair. The more legs the chair has, the more stable it is. A hydride shift is like adding a leg to the chair. If the shift creates a more stable carbocation, it’s like adding a leg to the chair, making it sturdier and more likely to stay upright.
In the biosynthesis of lanosterol, multiple hydride shifts happen to create a more stable carbocation in each step. This series of shifts is a fascinating example of how nature uses these rearrangements to build complex molecules. Understanding hydride shifts and carbocation stability is crucial for understanding organic chemistry reactions.
How do you know if it is a methyl or hydride shift?
A hydride is simply a hydrogen atom with an extra electron, giving it a negative charge. Methyl shifts involve the movement of an entire methyl group, which is a carbon atom bonded to three hydrogen atoms (CH3).
Now, how do we know which shift is happening? It all comes down to understanding what’s moving. Look for the movement of a single hydrogen atom – that’s a hydride shift. On the other hand, if a CH3 group is on the move, that’s a methyl shift.
Think of it like this: imagine a game of musical chairs where the players are atoms and molecules. In a hydride shift, the hydrogen atom (the player) just hops to a different chair. In a methyl shift, the whole methyl group (a team of players) moves together to a different chair.
Here’s a simple analogy:
Hydride shift: Imagine a single person moving from one seat to another.
Methyl shift: Imagine a whole family moving from one house to another.
Both shifts are important in organic chemistry, as they allow for the rearrangement of atoms within a molecule, often leading to the formation of new and different molecules.
Why is hydride shift preferred over methyl shift?
You’re right, a hydride shift (the movement of a hydrogen atom with its electrons) is generally more likely than a methyl shift (the movement of a CH3 group). However, the statement that a hydride shift *prefers* a methyl shift is a bit misleading. It’s not about preference, but rather about the stability of the resulting carbocations.
Hydride shifts usually lead to a more stable carbocation because they create a secondary carbocation, while methyl shifts often form a tertiary carbocation. Tertiary carbocations are more stable than secondary carbocations due to the electron-donating effect of the additional alkyl groups. This means, even though a hydride shift is a more common occurrence, the methyl shift might be preferred in specific situations where the formation of a tertiary carbocation outweighs the inherent favorability of the hydride shift.
Let’s break it down:
Hydride shift: This involves moving a hydrogen atom, usually leading to a secondary carbocation.
Methyl shift: This involves moving a methyl group (CH3), often leading to a tertiary carbocation.
Here’s the key: Tertiary carbocations are more stable because they are surrounded by more electron-donating groups (alkyl groups), which help stabilize the positive charge. This is where the concept of thermodynamic control comes in. The reaction will favor the formation of the more stable product, even if it requires a less likely shift.
In summary, while hydride shifts are generally favored due to their higher migratory aptitude, in cases where a methyl shift leads to a significantly more stable carbocation (like forming a tertiary carbocation), the thermodynamic control takes over, and the methyl shift becomes the dominant pathway.
How will you identify if shifts or 1/2 rearrangements will occur?
Let’s break it down:
1,2-Hydride Shifts: Think of it like a “hydrogen hand-off.” This happens when a secondary carbocation (a carbon with a positive charge and one other carbon attached) is next to a tertiary carbon (a carbon with a positive charge and three other carbons attached) that also has a hydrogen. The hydrogen essentially hops over to the secondary carbocation, creating a more stable tertiary carbocation.
1,2-Alkyl Shifts: Similar to the hydride shift, but instead of a hydrogen, an entire alkyl group (a group of carbons and hydrogens) migrates. This occurs when a secondary carbocation is next to a quaternary carbon (a carbon with a positive charge and four other carbons attached).
Why do these shifts happen? It’s all about stability. Carbocations are very reactive, and they want to become as stable as possible. Tertiary carbocations are more stable than secondary carbocations because they have more electron-donating groups attached to them, which helps to stabilize the positive charge.
Let me give you an example: Imagine a secondary carbocation next to a tertiary carbon with a hydrogen. The hydrogen on the tertiary carbon sees the unstable secondary carbocation and thinks, “I can help! I’ll move over to the secondary carbon and make it more stable.” This creates a more stable tertiary carbocation, and the reaction proceeds.
Remember: Look for those secondary carbocations next to tertiary or quaternary carbons. If you see them, there’s a good chance a shift will occur to create a more stable intermediate.
Does SN1 have a hydride shift?
Hydride shifts are definitely possible in SN1 reactions. They’re a way for a carbocation to rearrange and become more stable. Imagine a positively charged carbon atom (a carbocation) next to a carbon with a hydrogen attached. That hydrogen, along with its electrons, can jump over to the positively charged carbon. It’s like a little internal “rescue mission” for the unstable carbocation.
Here’s how it works:
1. The Leaving Group Leaves: The first step in an SN1 reaction is the departure of the leaving group. This creates a carbocation.
2. Hydride Shift (if possible): If the carbocation is unstable (usually primary or secondary), it can undergo a hydride shift. This moves the positive charge to a more stable position, often a tertiary carbon.
3. Nucleophile Attack: Once the carbocation is as stable as it can be, a nucleophile can attack it, forming a new bond.
Let’s look at an example:
Imagine a primary alkyl halide undergoing solvolysis (an SN1 reaction). When the leaving group leaves, you get a primary carbocation. This primary carbocation is very unstable. But, if there’s a hydrogen on a neighboring carbon, a hydride shift can occur. This shifts the positive charge to a secondary carbon, which is more stable.
Why does this happen? Because carbocations are more stable when they have more alkyl groups attached to them. Think of it like having more friends around – the more friends you have, the more stable you feel!
Important Note: Hydride shifts are a bit more likely to occur with primary and secondary carbocations than tertiary carbocations. Tertiary carbocations are already relatively stable, so they don’t need to rearrange as much.
So, to answer your question: Yes, hydride shifts can definitely occur in SN1 reactions. They’re a cool example of how molecules can rearrange themselves to become more stable!
Are there hydride shifts in E1?
Let’s break down how hydride shifts work in E1 reactions. A hydride shift is the migration of a hydrogen atom (H-) from an adjacent carbon to the carbocation, forming a new carbon-hydrogen bond and a new carbon-carbon bond. This process effectively moves the positive charge to a more stable position, usually to a carbon with more alkyl substituents.
Here’s why hydride shifts are important in E1 reactions:
Increased stability: By relocating the positive charge to a more substituted carbon, the carbocation becomes more stable. This stability is due to the electron-donating effect of alkyl groups, which helps to disperse the positive charge.
Altered product distribution: The rearrangement of the carbocation intermediate can change the final product distribution of the E1 reaction. This is because different carbocations can lead to the formation of different alkenes.
Hydride shifts are a critical aspect of understanding the mechanisms of E1 reactions, as they directly influence the product formation and overall reaction pathway. Keep in mind that the likelihood and type of rearrangement depend on the specific structure of the starting material and the reaction conditions.
See more here: Does Hydride Shift Occur In Sn2? | When Do Hydride Shifts Occur
What is hydride shift?
Here’s how it works:
Carbocation Formation: First, a carbocation is formed. This usually happens when a leaving group departs from a molecule.
Hydride Shift: The hydrogen atom, in the form of a hydride ion, then moves from an adjacent carbon atom to the carbon atom with the positive charge. This movement creates a new carbocation.
Stability: The new carbocation formed after the hydride shift is typically more stable than the original one. This is because it has a more even distribution of positive charge.
Think of it like this: Imagine a wobbly tower. The hydride shift is like moving a block from one side of the tower to another to make it more balanced. The new, more stable structure, is the balanced tower.
Let’s look at an example:
If you have a primary carbocation (the positive charge is on a carbon atom attached to only one other carbon atom), it’s generally not very stable. By undergoing a hydride shift, it can become a secondary carbocation (the positive charge is on a carbon atom attached to two other carbon atoms), which is more stable. This stability comes from the fact that the positive charge is shared among more carbon atoms in a secondary carbocation.
Hydride shifts are essential in many organic reactions, especially those involving rearrangements. They are also crucial in understanding the mechanisms of various reactions, including SN1 reactions and electrophilic aromatic substitutions.
Hydride shifts are driven by the desire for stability. Nature loves stability, and that’s why carbocations undergo these fascinating transformations.
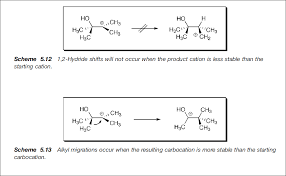
What would happen if a hydride shift occurred?
Imagine a carbocation – an ion with a positively charged carbon atom. If a hydride shift were to occur, the most likely place for it to happen would be from a carbon atom adjacent to the carbocation. This shift would result in the formation of a new carbocation, but this new carbocation would be less stable than the original one.
Think of it like this: Imagine you have a ball at the top of a hill, and you want to move it to a different position. You could move it to a lower point on the hill, which would be more stable, or you could move it to a higher point, which would be less stable. The hydride shift is like moving the ball to a higher point on the hill because it creates a less stable carbocation.
For example, let’s say we have a secondary carbocation (a carbocation with two carbon atoms attached to the positively charged carbon). If a hydride shift were to occur, it could move to an adjacent carbon, forming a primary carbocation (a carbocation with one carbon atom attached to the positively charged carbon). Since primary carbocations are less stable than secondary carbocations, this shift wouldn’t happen spontaneously.
Why is stability important? Well, in chemistry, things tend to move toward more stable states. Like a ball rolling down a hill, chemical reactions will often favor the formation of more stable molecules. So, while a hydride shift *could* happen, it’s not something you’d typically see in a reaction where it would result in a less stable carbocation.
Now, let’s look at why a hydride shift wouldn’t happen in this scenario. The hydride shift would create a less stable carbocation. You might be wondering why the original carbocation is more stable than the new one. The answer lies in the number of alkyl groups attached to the positively charged carbon. Alkyl groups are carbon-containing groups that release electrons. The more alkyl groups attached to a carbocation, the more electron density around the positive charge, and the more stable the carbocation.
So, in the case of our secondary carbocation, we have two alkyl groups attached to the positively charged carbon. By shifting the hydride, we’re moving the positive charge to a carbon with only one alkyl group attached. This leads to a less stable primary carbocation. In essence, the original secondary carbocation is more stable because it’s more surrounded by electron-donating alkyl groups.
While a hydride shift *could* occur, it’s important to remember that it’s a thermodynamic process. The reaction will favor the formation of the more stable product. In this case, the secondary carbocation is more stable, so the hydride shift wouldn’t happen.
What are the preceeding two possible hydride shifts?
We’re specifically interested in the two possibilities that came before the 1,3 hydride shift you mentioned.
Since hydride shifts involve the movement of a hydride ion (H-) from one carbon to another, we can identify the previous possibilities based on the positions of the carbons involved.
The two possibilities before the 1,3 hydride shift were 1,2 hydride shifts.
These shifts occur between adjacent carbons, meaning the hydride ion moves from one carbon to the carbon right next to it. This kind of shift is the most common type of hydride shift because it typically leads to a more stable carbocation.
1,2 hydride shifts are crucial in understanding how molecules rearrange themselves during chemical reactions. Imagine a carbocation – a carbon with a positive charge. This positive charge wants to be as stable as possible, and a hydride shift can help it achieve that.
Think of it like this: The hydride ion (H-) is like a “helper” for the carbocation. By moving to the adjacent carbon, it essentially “shares” its electron with the positively charged carbon, creating a more stable arrangement.
Now, you mentioned that 1,3 hydride shifts are also possible, but they are less common. In this scenario, the hydride ion moves from a carbon two positions away from the original carbocation. This movement can also create a more stable carbocation, but it usually happens when a 1,2 hydride shift is not possible or when it leads to a less stable carbocation.
Understanding the different types of hydride shifts is essential in understanding the mechanisms of many chemical reactions. It helps us predict what products we might get from a specific reaction and how the molecules might rearrange themselves.
How hydride shift occurs in a carbocation reaction?
You know how carbocations are formed when a leaving group departs, leaving behind a positively charged carbon? Well, these carbocations can sometimes rearrange themselves to become more stable. One way they do this is through a hydride shift. Imagine a hydrogen atom with its two electrons, like a little package, moving from one carbon to a neighboring carbon. This is exactly what happens in a hydride shift.
So, why would a carbocation want to do this? It’s all about achieving the most stable structure. Carbocations prefer to have as many alkyl groups as possible attached to the positively charged carbon. This is because alkyl groups are electron-donating, which helps to stabilize the positive charge.
Let’s take an example. Imagine you have a primary carbocation (a carbocation with only one alkyl group attached). This carbocation isn’t very stable. Now, if you have a neighboring carbon that has a hydrogen atom, that hydrogen can shift over to the positively charged carbon, forming a secondary carbocation (a carbocation with two alkyl groups attached). This secondary carbocation is more stable than the primary carbocation.
Hydride shifts are commonly seen in reactions involving alcohols and hydrogen halides, such as HBr, HCl, and HI. In these reactions, the alcohol undergoes a nucleophilic substitution reaction, where the hydrogen halide acts as the nucleophile. During this process, a carbocation intermediate is formed, and it might undergo a hydride shift to attain a more stable structure.
Think of it like this: a hydride shift is a way for a carbocation to shuffle its electrons around to get into a more comfortable, more stable configuration. It’s like rearranging the furniture in your living room to make it feel more spacious and inviting. The carbocation is just trying to find its own comfortable position!
See more new information: linksofstrathaven.com
When Do Hydride Shifts Occur: Understanding Carbocation Rearrangements
Alright, let’s dive into the fascinating world of hydride shifts in organic chemistry. You might be thinking, “Hydride shifts? What are those?” Don’t worry, I’ll break it down for you.
Essentially, a hydride shift is a type of rearrangement reaction where a hydride ion, which is basically a hydrogen atom with two electrons, moves from one atom to another within the same molecule. This movement, this shift, is crucial for understanding how certain organic reactions proceed.
So, when exactly do these hydride shifts happen? Well, it all boils down to stability. Hydride shifts occur to create a more stable carbocation. Let’s explain what that means.
Carbocations are positively charged carbon atoms. They’re not too happy about having that positive charge. They want to get rid of it as quickly as possible. And the way they do that is by shifting around electrons and atoms, trying to find the most stable configuration.
Think of it like this: imagine a wobbly chair. It’s unstable, and it wants to find a more stable position. A hydride shift is like that chair finding a sturdier base.
Now, there are a few key factors that influence whether or not a hydride shift will happen:
The stability of the starting carbocation: If the starting carbocation is already pretty stable, a hydride shift is less likely. It’s like that chair that’s already pretty stable; it doesn’t really need to move.
The stability of the resulting carbocation: If the hydride shift leads to a more stable carbocation, it’s more likely to happen. It’s like that wobbly chair finding a much sturdier base; it’s definitely going to make the move.
The type of reaction: Certain reactions, like SN1 reactions and electrophilic addition reactions, are more likely to involve hydride shifts.
So, how do we determine the stability of a carbocation? Well, there are a few rules of thumb:
More substituted carbocations are more stable: This means that carbocations with more alkyl groups attached to the positive carbon are more stable. Tertiary carbocations are the most stable, followed by secondary carbocations, and then primary carbocations.
Resonance stabilization: If the positive charge can be delocalized by resonance, the carbocation will be more stable. Think of it like spreading the positive charge out over a larger area, making it less concentrated and thus more stable.
Let’s look at some examples to solidify this:
Example 1: SN1 Reaction
In an SN1 reaction, the leaving group leaves first, forming a carbocation. If the carbocation is unstable, it might undergo a hydride shift to become more stable.
For instance, if we have a primary carbocation, it might undergo a hydride shift to become a more stable secondary carbocation.
Example 2: Electrophilic Addition
In electrophilic addition, a carbocation intermediate is formed. Again, if this carbocation is unstable, a hydride shift might occur to create a more stable carbocation.
Imagine the addition of HBr to an alkene. The initial carbocation formed might undergo a hydride shift to produce a more stable tertiary carbocation.
Hydride shifts can be a bit tricky to wrap your head around at first. But once you understand the basic principles of carbocation stability and how hydride shifts work to increase that stability, it’ll all start to make sense.
Let me know if you have any other questions. I’m here to help!
FAQs
1. What is a hydride shift?
A hydride shift is a type of rearrangement reaction where a hydride ion (H-) moves from one atom to another within the same molecule.
2. Why do hydride shifts occur?
Hydride shifts occur to create a more stable carbocation.
3. How do we determine the stability of a carbocation?
Carbocation stability is influenced by the number of alkyl groups attached to the positively charged carbon, as well as resonance stabilization.
4. What types of reactions are hydride shifts common in?
Hydride shifts are common in SN1 reactions and electrophilic addition reactions.
5. Are there any other types of shifts in organic chemistry?
Yes, there are other types of shifts, such as methyl shifts and alkyl shifts.
6. How do I know when a hydride shift will occur?
Consider the stability of the starting carbocation and the potential stability of the resulting carbocation after the shift. If the shift leads to a more stable carbocation, it’s more likely to happen.
7. Can you give me a real-world example of where hydride shifts are important?
Hydride shifts are crucial in many biological reactions, like the metabolism of carbohydrates.
8. What are some common misconceptions about hydride shifts?
One common misconception is that hydride shifts always occur. This isn’t true; hydride shifts only happen when they lead to a more stable carbocation.
9. What resources can I use to learn more about hydride shifts?
You can check out your organic chemistry textbook, online resources like Khan Academy, or YouTube videos on the topic.
10. Can you recommend any books on this topic?
“Organic Chemistry” by Paula Yurkanis Bruice is a great textbook for learning about hydride shifts and other organic chemistry concepts.
Carbocation Rearrangements – Chemistry LibreTexts
Typically, hydride shifts can occur at low temperatures. However, by heating the solutionf of a cation, it can easily and readily speed the process of rearrangement. One way to account for a slight barrier is to propose a 1,3-hydride shift Chemistry LibreTexts
Rearrangement Reactions (1) – Hydride Shifts
How to spot a substitution with a hydride shift, how to know when a hydride shift will occur, with examples, mechanisms, and more. Master Organic Chemistry
Hydride Shift – Introduction, Reaction Mechanism, Reaction
A hydride shift will occur whenever we have a more substituted carbon around a cationic carbon. It would shift the positive charge over to carbon, stabilising it better, BYJU’S
7.10: Rearrangements of the Carbocation and Sₙ1 Reactions
Hydride Shift. The hydride shift can also be called the 1,2-Hydride Shift because rearrangements primarily occur between adjacent carbon atoms. The 1,2 are Chemistry LibreTexts
Hydride Shift | Orgoreview
Hydride shift is a type of rearrangement reaction in which a hydrogen atom in a carbocation rearranges (shifts) its position to change that carbocation to more stable carbocation. Orgoreview
Carbocation stability and rearrangement introduction
So, a hydride shift could occur to form a more stable carbocation. So, let’s look at this carbocation here. We know that this carbon has the plus one formal charge. Khan Academy
E1 mechanism: carbocations and rearrangements – Khan Academy
The preceeding two possibilities are 1,2 hydride shifts since they happen between adjacent carbons on the chain. It is however possible for a 1,3 hydride shift to occur where a hydride from a carbon two spots away on the chain from the original Khan Academy
Sn1 mechanism: carbocation rearrangement (video) | Khan
Methyl and hydride shifts are pretty fast compared to a nucleophilic attack because they’re intermolecular rearrangements that don’t require any collisions to occur. A secondary carbocation is stable enough that you’ll get a mixture of the two products. Khan Academy
Hydride Shift and Methyl Shift Mechanism – Leah4sci
Hydride Shift and Methyl Shift Mechanism. Hydride shifts and methyl shifts can occur in organic chemistry reactions if there is a carbocation intermediate. Leah4Sci
Sn1 Carbocation Rearrangements – Hydride Shift \U0026 Methyl Shift
Hydride Shift And Methyl Shift – Carbocation Rearrangements | Organic Chemistry
Hydride Shift Vs Methyl Shift – Carbocation Rearrangement
Carbocation Rearrangement – Hydride And Methanide Shifts
Hydride Shift And Methyl Shift Mechanism
Hydride Shifts In Sn1
Hydride Shifts And Methyl Shifts
Link to this article: when do hydride shifts occur.
See more articles in the same category here: https://linksofstrathaven.com/how
