Does chlorobutane react with silver nitrate?
The reaction you mentioned, chloroethane with silver nitrate, is actually a little bit different.Chloroethane reacts with silver nitrate to form ethyl nitrate and silver chloride. Let’s break this down:
Chloroethane is an alkyl halide with the formula CH3CH2Cl.
Silver nitrate is a common inorganic salt with the formula AgNO3.
When you mix these two compounds, a substitution reaction occurs. Silver ions (Ag+) from the silver nitrate replace the chlorine atom in chloroethane. This results in the formation of ethyl nitrate (CH3CH2ONO2) and silver chloride (AgCl), which is a white precipitate that often forms in this reaction.
Now, let’s talk about chlorobutane. Chlorobutane is an alkyl halide with the formula CH3CH2CH2CH2Cl. You might be wondering, if chloroethane reacts with silver nitrate, will chlorobutane also react? The answer is yes!
Chlorobutane will react with silver nitrate in a similar way to chloroethane. The silver ions will replace the chlorine atom in chlorobutane to form butyl nitrate (CH3CH2CH2CH2ONO2) and silver chloride (AgCl).
The reaction of chlorobutane with silver nitrate is also a substitution reaction. This type of reaction occurs because silver ions are strong electrophiles, meaning they’re attracted to electron-rich species, and the chlorine atom in chlorobutane is a good leaving group, which means it’s easily displaced.
So, the bottom line is that both chloroethane and chlorobutane will react with silver nitrate, but they will produce different products. Both reactions are good examples of substitution reactions involving alkyl halides and silver ions.
This kind of reaction is often used in organic chemistry to synthesize different organic compounds. It’s also a great way to study the reactivity of halogenalkanes and silver salts.
What chemicals react with silver nitrate?
If you place a copper rod in a silver nitrate solution and let it sit for a while, you’ll see something really cool happen. The silver nitrate will react with the copper, forming silver crystals that look like hair! At the same time, the solution will turn blue due to the formation of copper nitrate. This reaction is represented by the following chemical equation:
2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
This reaction is a classic example of a single displacement reaction. In this type of reaction, a more reactive element (in this case, copper) displaces a less reactive element (silver) from its compound.
The silver crystals form because copper is more reactive than silver, meaning it has a stronger tendency to lose electrons. As the copper atoms lose electrons, they form copper ions (Cu2+), which then dissolve in the solution, creating the blue color. Meanwhile, the silver ions (Ag+) in the solution gain electrons from the copper and become silver atoms (Ag), which then precipitate out of the solution as shiny, hair-like crystals.
The reaction between silver nitrate and copper is a visually striking demonstration of the principles of reactivity and displacement reactions. It’s a great way to learn about how different elements interact with each other.
Why would the reaction of 2-chlorobutane with silver nitrate in ethanol proceed at a slower rate than the reaction of 2-chloro-2-methylpropane with silver nitrate in ethanol?
Let’s break down why this is the case. The reaction with silver nitrate involves an SN1 reaction, a two-step process. The first step is the formation of a carbocation. The stability of this carbocation determines the rate of the reaction. A more stable carbocation forms faster, leading to a faster overall reaction.
Now, why is the carbocation formed from 2-chloro-2-methylpropane more stable? It’s because of the inductive effect of the three methyl groups attached to the central carbon. These methyl groups are electron-donating, meaning they push electron density towards the positively charged carbon, stabilizing the carbocation.
In 2-chlorobutane, the positive charge on the carbon is only stabilized by the inductive effect of one methyl group. This makes the carbocation less stable and therefore harder to form, slowing down the overall reaction. In essence, the more alkyl groups attached to the carbon bearing the positive charge, the more stable the carbocation and the faster the SN1 reaction.
Does 2-bromobutane react with silver nitrate?
You’re right, 2-bromobutane does react with silver nitrate, but it’s a bit more nuanced than simply saying it gives a “positive test.”
Here’s what happens:
2-bromobutane will react with silver nitrate in the presence of a suitable solvent, like ethanol, to form a precipitate of silver bromide. This reaction is a classic example of a nucleophilic substitution reaction.
Silver nitrate is a source of silver ions, which act as electrophiles. 2-bromobutane has a polar carbon-bromine bond, making the carbon atom slightly electrophilic. The silver ion attacks the carbon atom, displacing the bromide ion.
The bromide ion then reacts with another silver ion to form silver bromide which precipitates out as a white solid. This precipitate formation is the key indicator of a positive test.
Now, why does this reaction happen? Well, it’s because bromide ions are good leaving groups. They are relatively stable on their own and are readily displaced by other nucleophiles, like the silver ion.
So, to summarize, 2-bromobutane reacts with silver nitrate in a nucleophilic substitution reaction, forming a precipitate of silver bromide. This precipitate is the key sign of a positive test.
Let’s clarify a few things from your original statement:
2-chlorobutane also reacts with silver nitrate, and you are correct to mention it, but that was a bit off-topic.
KI with acetone is a separate reaction altogether. While interesting, it wasn’t relevant to the original question.
Focus on the main point – 2-bromobutane reacts with silver nitrate and that’s the information we want to highlight.
Which does not react with AgNO3?
This is because the chloride ions in PtCl₂·2NH₃ are tightly bound to the platinum atom, forming a complex ion. This complex ion is very stable, meaning that the chloride ions are not readily available to react with the silver ions in AgNO₃.
Think of it like this: the chloride ions are locked in a cage with the platinum, making it impossible for them to escape and react with the silver ions. This is a classic example of a coordination compound, where a central metal ion is surrounded by ligands, which are molecules or ions that donate electrons to the metal. In this case, the platinum is the central metal ion and the chloride ions and ammonia molecules are the ligands.
The stability of this complex ion is due to several factors, including:
The strong bond between the platinum and the chloride ions. This bond is very strong because it involves a combination of ionic and covalent bonding.
The presence of ammonia ligands. These ligands further stabilize the complex ion by donating electron pairs to the platinum.
In contrast, if we had a compound like NaCl, the chloride ions would be readily available to react with AgNO₃, forming a white precipitate of AgCl. This is because the chloride ions are not bound to a central metal ion in NaCl.
So, to summarize, the reason why PtCl₂·2NH₃ does not react with AgNO₃ is due to the formation of a stable complex ion, where the chloride ions are tightly bound to the platinum atom and are not available to react with the silver ions.
What happens when Cu reacts with AgNO3?
Here’s how it works: Copper is more reactive than silver, meaning it has a stronger tendency to lose electrons. When you combine copper with silver nitrate, the copper atoms will displace the silver atoms in the silver nitrate solution.
The copper atoms give up electrons and form copper nitrate (Cu(NO3)2), which dissolves in the water. The silver atoms, now free, will solidify out of the solution as silver metal.
The equation for this reaction is:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Here’s a visual breakdown:
Copper (Cu) is a reddish-brown metal.
Silver nitrate (AgNO3) is a colorless solution.
Copper nitrate (Cu(NO3)2) is a blue-green solution.
Silver (Ag) is a shiny, white metal.
What happens in the reaction:
1. Copper is placed in a silver nitrate solution.
2. Copper is more reactive than silver and displaces it.
3. Copper reacts with nitrate ions to form copper nitrate, a soluble compound.
4. Silver atoms are displaced from the solution and precipitate out as solid silver.
You’ll notice some key changes:
* The solution will turn blue-green, indicating the formation of copper nitrate.
Silver will begin to form as a shiny, metallic coating on the copper or may even fall to the bottom of the container as a solid.
This reaction demonstrates the concept of reactivity series, which helps us predict which metal will displace another in a reaction. Copper is higher on the reactivity series than silver, meaning it is more likely to lose electrons and react.
Let’s get a little deeper:
This reaction is a great example of how we can use our knowledge of chemistry to understand and predict how different substances will behave. By understanding the reactivity series, we can predict the outcome of many different chemical reactions.
What compounds react with AgNO3?
When you mix an aqueous solution of Co(NH3)5Br(SO4) with AgNO3, a yellowish-white precipitate forms. This precipitate is silver bromide (AgBr).
The reaction happens because AgNO3 is a good source of silver ions (Ag+). These silver ions react with the bromide ions (Br-) present in the complex compound Co(NH3)5Br(SO4). Silver bromide (AgBr) is insoluble in water, so it precipitates out as a solid.
Here’s a breakdown of the reaction:
Co(NH3)5Br(SO4) is a complex compound where the cobalt ion (Co3+) is coordinated with five ammonia molecules (NH3), one bromide ion (Br-), and one sulfate ion (SO42-).
AgNO3 readily dissolves in water to give silver ions (Ag+) and nitrate ions (NO3-).
* When these two solutions are mixed, the Ag+ and Br- ions react to form the precipitate AgBr.
The key takeaway is that this reaction showcases the ability of silver ions (Ag+) from silver nitrate (AgNO3) to displace bromide ions (Br-) from a complex compound, forming a precipitate of silver bromide (AgBr).
Let’s expand on this to gain a deeper understanding of what’s happening. Co(NH3)5Br(SO4) is a coordination complex. Coordination complexes are formed when a central metal ion (in this case, cobalt) is surrounded by ligands (molecules or ions that donate electrons to the metal). The ligands in Co(NH3)5Br(SO4) are ammonia molecules (NH3), bromide ions (Br-), and a sulfate ion (SO42-).
The reaction with AgNO3 highlights a key characteristic of coordination complexes: the ligands can be replaced by other ions or molecules. In this case, the bromide ion (Br-) is replaced by a silver ion (Ag+), forming AgBr.
This type of reaction, where a ligand is replaced, is called a ligand substitution reaction. These reactions are common in coordination chemistry and are important for various applications, such as catalysis and the synthesis of new materials.
Understanding the reactivity of coordination complexes with compounds like AgNO3 is crucial for understanding a wide range of chemical processes and applications.
What neutralizes silver nitrate?
Silver nitrate is a chemical compound that is used as an antiseptic and a cauterizing agent. It is also used in the production of some medications. Silver nitrate can be harmful if it comes into contact with the skin, eyes, or mucous membranes. If you come into contact with silver nitrate, it is important to flush the area with water immediately.
Normal saline is a solution of salt and water that is similar to the fluid found in our bodies. It is used to flush wounds, to irrigate the eyes, and to neutralize the effects of certain chemicals, including silver nitrate.
Normal saline works by diluting the silver nitrate and preventing it from reacting with the skin. It also helps to remove any remaining silver nitrate from the skin.
You should always consult with a doctor or pharmacist before using silver nitrate or normal saline. They can help you determine the best way to use these products and can provide advice on how to prevent any potential side effects.
See more here: What Chemicals React With Silver Nitrate? | Does 2-Chlorobutane React With Silver Nitrate
Is 2-chlorobutane a SN1 reaction?
Let’s break down why the solvent’s polarity affects the reaction. SN1 reactions occur in two steps. The first step is the formation of a carbocation, which is a positively charged carbon atom. This step is slow and rate-determining. The second step is the attack of the nucleophile on the carbocation, which is fast.
A polar solvent, like water or ethanol, helps to stabilize the carbocation intermediate. This happens because the polar solvent molecules surround the carbocation, shielding it from the negative charges of other molecules. This stabilization lowers the energy of the carbocation, making it easier to form and increasing the rate of the SN1 reaction.
In the case of 2-chlorobutane, the solvent mixture of ethanol and water was less effective because the reaction required heat. This implies that the carbocation was not as stable as it would be in a more polar solvent. The heat provided the necessary energy to overcome the energy barrier for the formation of the carbocation.
In essence, the use of heat suggests that the solvent mixture was not sufficiently polar to fully stabilize the carbocation intermediate, thus slowing down the rate of the SN1 reaction. A more polar solvent would have made the reaction proceed faster at room temperature.
How do halogenoalkanes react with silver nitrate?
We’re going to mix different halogenoalkanes with a solution of silver nitrate in a mixture of ethanol and water. This is a neat experiment because we can observe a change – precipitates form! These precipitates are formed when the halide ions released from the halogenoalkanes react with the silver ions present in the solution.
Here’s the breakdown:
1. Halogenoalkanes (also known as haloalkanes) are organic compounds where one or more hydrogen atoms are replaced by halogen atoms (like chlorine, bromine, or iodine).
2. When we add these halogenoalkanes to the silver nitrate solution, the halogenoalkanes undergo a nucleophilic substitution reaction. This means that the halogen atom is replaced by a hydroxyl group (OH-) from the water molecules.
3. This reaction releases halide ions (Cl-, Br-, or I-) into the solution.
4. These halide ions then react with the silver ions (Ag+) from the silver nitrate, forming an insoluble silver halide precipitate.
* Silver chloride (AgCl) is a white precipitate.
* Silver bromide (AgBr) is a cream precipitate.
* Silver iodide (AgI) is a yellow precipitate.
The rate at which these precipitates form depends on the type of halogen atom in the halogenoalkane. The carbon-halogen bond strength plays a crucial role. For example, chloroalkanes (halogenoalkanes with chlorine) react slower than bromoalkanes (halogenoalkanes with bromine) due to the stronger carbon-chlorine bond.
This reaction with silver nitrate is a useful test to distinguish between different types of halogenoalkanes. By observing the color of the precipitate and the speed at which it forms, we can identify the type of halogen present in the original halogenoalkane.
Can silver nitrate be used to test for halogenoalkane?
Let’s break down the process. First, you need to understand that halogenoalkanes are organic compounds where one or more hydrogen atoms are replaced by halogen atoms like chlorine, bromine, or iodine. When you react a halogenoalkane with an aqueous solution of silver nitrate, a substitution reaction occurs, replacing the halogen with a nitrate group. This reaction produces a silver halide precipitate, which can be used to identify the original halogen present in the halogenoalkane.
The beauty of this reaction lies in the distinct colors of the silver halide precipitates. Silver chloride (AgCl) is white, silver bromide (AgBr) is cream, and silver iodide (AgI) is yellow. This color difference allows us to quickly identify the halogen present in the original halogenoalkane. For instance, if a white precipitate forms when silver nitrate is added, we know that the halogenoalkane contained chlorine. Similarly, a cream precipitate indicates bromine, and a yellow precipitate indicates iodine.
However, it’s important to remember that the reaction with silver nitrate solution is not always immediate. The rate of the substitution reaction depends on the type of halogenoalkane and the reaction conditions. Sometimes, you might need to heat the mixture or add a catalyst to speed up the reaction and facilitate the formation of a precipitate.
Here’s a simple breakdown:
1. The reaction: Halogenoalkane + AgNO3 (aq) → AgX (s) + other products (X represents the halogen)
2. The precipitate: The precipitate formed (AgX) is a silver halide with a distinct color depending on the halogen (chlorine = white, bromine = cream, iodine = yellow).
This simple test, using silver nitrate solution, provides a quick and reliable way to identify the presence of halogens in halogenoalkanes.
See more new information: linksofstrathaven.com
Does 2-Chlorobutane React With Silver Nitrate | Does Chlorobutane React With Silver Nitrate?
The Reaction: A Look at the Fundamentals
First things first, 2-chlorobutane is an alkyl halide. This means it has a carbon-halogen bond, specifically, a carbon-chlorine bond in this case. Silver nitrate, on the other hand, is a classic reagent used to test for the presence of halides.
Now, the question is: will 2-chlorobutane react with silver nitrate? The answer is, yes, but with a catch. This reaction needs a little help to happen, and that help comes in the form of a polar solvent, such as ethanol.
Let’s break down why this reaction happens. When 2-chlorobutane is mixed with silver nitrate in the presence of ethanol, a nucleophilic substitution reaction takes place. This reaction involves the exchange of a leaving group, the chlorine atom in this case, with a nucleophile, which is the nitrate ion (NO3-) from silver nitrate.
Here’s the step-by-step explanation:
1. Solvation: The ethanol solvent helps solvate both the 2-chlorobutane and the silver nitrate, making them more reactive.
2. Nucleophilic Attack: The nitrate ion, a good nucleophile, attacks the carbon atom bonded to the chlorine atom in 2-chlorobutane.
3. Leaving Group Departure: The chlorine atom, being a good leaving group, departs as a chloride ion (Cl-).
4. Product Formation: The final product is 2-nitrobutane and silver chloride precipitate.
The silver chloride precipitate is a key indicator that the reaction has occurred. It’s a white solid that forms a cloudy suspension in the solution. This is a classic test used to identify the presence of halides.
Factors Affecting the Reaction
Several factors can influence the rate and efficiency of this reaction.
1. Temperature: Increasing the temperature generally speeds up the reaction. This is because higher temperatures increase the kinetic energy of the molecules, leading to more frequent and energetic collisions between the reactants.
2. Solvent Polarity: Using a more polar solvent can enhance the reaction rate. This is due to better solvation of the reactants and the stabilization of the transition state during the reaction.
3. Concentration: Higher concentrations of both reactants can lead to a faster reaction. This is simply because there are more opportunities for collisions between the molecules.
4. Nature of the Halide: The type of halogen atom attached to the carbon atom can impact the reaction rate. For instance, a bromine atom would be more readily displaced than a chlorine atom because it’s a better leaving group.
Why It Matters
Understanding this reaction is crucial in various fields, especially in:
1. Organic Synthesis: It provides a method for synthesizing nitroalkanes, which are important compounds in various applications, including the production of explosives and pharmaceuticals.
2. Analytical Chemistry: The reaction forms the basis of halide tests, allowing us to identify and quantify the presence of halides in different samples.
FAQs
What if we don’t have ethanol?
While ethanol is the go-to solvent for this reaction, you can try using other polar solvents like acetone or dimethylformamide (DMF). However, the reaction rate and yield might be different.
Can we use other silver salts?
Yes, you can try using other silver salts, but the reaction mechanism and the product will be different. For instance, using silver fluoride would likely result in the formation of 2-fluorobutane, a different product.
What if we use a different alkyl halide?
The reaction will proceed similarly with other primary and secondary alkyl halides, but the rate of reaction will be different depending on the nature of the halide and the structure of the alkyl group.
Can this reaction be used to synthesize other types of organic compounds?
Yes, the SN2 reaction, the type of reaction we discussed, is a versatile method for synthesizing various organic compounds. By changing the nucleophile, you can get different products.
What are the safety precautions?
2-chlorobutane is a volatile and flammable substance, so it should be handled with care in a well-ventilated area. Silver nitrate can cause skin and eye irritation, so it’s important to wear appropriate safety gear, including gloves and goggles.
By understanding the reaction between 2-chlorobutane and silver nitrate, you gain insight into the fundamental principles of organic chemistry, paving the way for further exploration of this fascinating field.
halogenoalkanes (haloalkanes) and silver nitrate – chemguide
Silver nitrate solution can be used to find out which halogen is present in a suspected halogenoalkane. The most effective way is to do a substitution reaction which turns the halogen into a halide ion, and then to test for that ion with silver nitrate solution. chemguide
Nucleophilic Substitution: Chemistry Lab – Odinity
The final reaction with 2-chlorobutane and 1% silver nitrate in a 1:1 mixture of ethanol and water was a SN1 reaction, but since the precipitate formed only with heat, odinity.com
Predicting order of nucleophilic substitution reactivity
For the $\ce{NaI}$ reaction, tertiary halides should react fastest and primary halides should react slowest. What order of reactivity do you predict will b observed when each alkyl Chemistry Stack Exchange
Chem 2219: Exp#7 Relative Rates of SN1 & SN2 Reactions
In this experiment we will look at how changing the structure of the substrate and the nature of the leaving group affects the relative rates for SN1 and SN2 reactions. The test mst.edu
3.3.2 Reactions of Halogenoalkanes – Save My Exams
Acidified silver nitrate can be used to measure the rate of hydrolysis of halogenoalkanes; Set up three test tubes in a 50 o C water bath, with a mixture of ethanol and acidified savemyexams.com
3.3.2 Substitution Reactions of Halogenoalkanes – Save My Exams
This reaction is classified as a nucleophilic substitution reaction with water molecules in aqueous silver nitrate solution acting as nucleophiles, replacing the halogen in the savemyexams.com
Reaction of Alkyl Halides with Silver Nitrate – Chemistry LibreTexts
The most effective way is to do a substitution reaction which turns the halogen into a halide ion, and then to test for that ion with silver nitrate solution. The Chemistry LibreTexts
6.4D: Individual Tests – Chemistry LibreTexts
Silver Nitrate Test. A dilute solution of silver nitrate in ethanol is a test for some alkyl halides. Silver has a high affinity for halogens (forms strong \(\ce{AgX}\) ionic bonds), Chemistry LibreTexts
2-Chlorobutane – Wikipedia
Synthesis. 2-Chlorobutane can be synthesized through the addition of hydrochloric acid to 2-butene in the following reaction: The reaction is two-step, with the pi electrons Wikipedia
STRUCTURE-REACTIVITY RELATIONSHIPS: NUCLEOPHILIC
Repeat the above procedures using a CLEAN, DRY set of test tubes using 1 mL of the silver nitrate solution as the reaction medium. Remember to shake the test tubes to ucalgary.ca
Reactions That Undergo Sn1- 2-Chlorobutane +Silver Nitrate In Ethanol 1- Bromobutane +Silver Nitrat…
Test For Chloride And Bromide Ions Using Silver Nitrate
Sodium Chloride And Silver Nitrate
Redox Reaction: Holiday Chemistree! Copper + Silver Nitrate (Holiday Chemistry)
Copper Wire And Silver Nitrate
What Happens When Copper Is Added To Silver Nitrate Solution
Copper Penny Reaction With Silver Nitrate (Cu + Agno3)
Link to this article: does 2-chlorobutane react with silver nitrate.
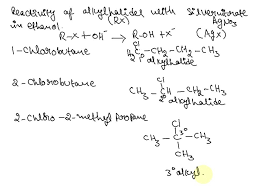
See more articles in the same category here: https://linksofstrathaven.com/how
