Where do electrons move around the nucleus?
The Bohr model is a helpful starting point for understanding the structure of atoms. It imagines electrons as tiny particles orbiting the nucleus in fixed, circular paths. This model is based on the idea that electrons have a specific energy level, and they can jump between these levels by absorbing or emitting light.
However, the Bohr model is limited. It doesn’t fully explain how electrons actually behave in atoms. The quantum mechanical model provides a more accurate and complex description of electron behavior. This model uses wave functions to describe the probability of finding an electron in a particular region of space around the nucleus.
Think of it like this: the Bohr model is like a simplified map of a city, showing the main roads and landmarks. It’s useful for getting a basic idea of the layout but doesn’t capture all the details. The quantum mechanical model is like a detailed map, showing every street, building, and park. It’s more accurate but also more complex.
The quantum mechanical model shows that electrons don’t have fixed paths like in the Bohr model. Instead, they exist in orbitals, which are three-dimensional regions of space where there’s a high probability of finding an electron. Each orbital has a specific shape and energy level.
For example, the s orbital is spherical and has the lowest energy level. The p orbitals are dumbbell-shaped and have a slightly higher energy level. There are even more complex shapes for higher energy levels.
Understanding how electrons move around the nucleus is essential for understanding the behavior of atoms and molecules. It explains why elements have different properties, how chemical bonds form, and ultimately how the world around us works.
Where are electrons arranged around the nucleus?
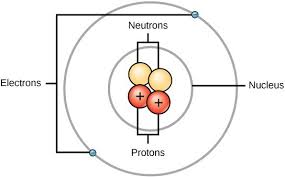
Shells are like containers that hold a specific number of electrons. The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell can hold up to eight electrons, and so on.
You can imagine these shells as concentric circles around the nucleus, with each circle representing a different energy level. The electrons in each shell are constantly moving around the nucleus, but they are restricted to their specific energy level.
Think of it like a solar system, where the nucleus is the sun and the electrons are planets orbiting the sun. Each planet orbits at a specific distance from the sun, which is analogous to the energy level of the electrons in an atom.
This organization of electrons into shells helps to explain the chemical properties of elements and how atoms bond with each other. Each element has a unique arrangement of electrons in its shells, which determines how it will interact with other atoms.
What is the location where electrons are found orbiting the nucleus?
We often picture electrons orbiting the nucleus like planets around the sun. While this is a helpful starting point, it’s not entirely accurate. Electrons don’t follow fixed paths. Instead, they exist in energy levels. Imagine these energy levels like steps on a ladder, with lower steps representing lower energy. Electrons at lower energy levels are closer to the nucleus and have less energy than those at higher energy levels.
Now, let’s dive a bit deeper into these orbitals. They’re not just random fuzzy clouds, but have specific shapes determined by their energy levels and the number of electrons they hold. These shapes are called atomic orbitals.
s orbitals are spherical, with the nucleus at the center.
p orbitals are dumbbell-shaped, with two lobes on either side of the nucleus.
d orbitals and f orbitals have more complex shapes, but their general idea is the same: they represent the probability of finding an electron in a specific region around the nucleus.
It’s important to remember that these orbitals aren’t rigid boundaries. They’re more like probabilistic maps, indicating where an electron is most likely to be found at any given time. This is due to the wave-particle duality of electrons, meaning they act like both waves and particles.
Thinking about orbitals helps us understand the behavior of atoms and how they interact with each other to form molecules. It’s a fascinating world of quantum mechanics, and understanding orbitals is a key step in unraveling the secrets of the universe!
Why do electrons spin around the nucleus?
The nucleus, as you said, is heavy and positively charged. Electrons are light and negatively charged. This attraction is what keeps the electrons bound to the atom. However, it’s not a simple case of orbiting.
Think of it this way: Imagine a tiny ball bearing spinning around a much larger ball. The ball bearing is constantly moving and changing direction, but it never actually hits the larger ball. This is similar to what happens with electrons.
Electrons have a specific amount of energy that dictates their behavior. They don’t just orbit the nucleus in a circular path. Instead, they exist in specific energy levels or orbitals. These orbitals are not like the orbits of planets. They are more like clouds that represent the probability of finding an electron in a specific region of space.
You can think of these orbitals as having different shapes and sizes, kind of like a fuzzy ball rather than a perfect sphere. The electron can be found anywhere within this fuzzy ball, but it’s more likely to be found in certain areas.
The quantum nature of electrons is what prevents them from spiraling into the nucleus. Imagine if a tiny ball bearing had a fixed amount of energy that allowed it to spin around a larger ball without hitting it. This is similar to how electrons maintain their specific energy levels. They can jump to a higher or lower energy level, but they can’t lose their energy completely and fall into the nucleus.
This is just a simplified explanation of a complex subject, but hopefully it helps you understand the basic principles of why electrons don’t just fall into the nucleus. The quantum nature of electrons and their specific energy levels play a crucial role in maintaining the structure of atoms.
Where are electrons revolving around the nucleus?
Think of it like this: Imagine a tiny, super-dense nucleus at the center of an atom, and the electrons are like tiny, buzzing flies that can only exist at certain distances from the nucleus. These distances correspond to the different energy levels.
Each shell has a specific amount of energy associated with it, and the electrons within a shell can move around within that shell, but they can’t jump to another shell without gaining or losing energy.
For example, if an electron absorbs a certain amount of energy, it can jump to a higher energy level (further away from the nucleus). If it loses energy, it can drop down to a lower energy level (closer to the nucleus).
Now, we can’t really know the exact location of an electron at any given moment because they’re constantly moving and their position is uncertain. Instead, we can talk about the probability of finding an electron in a particular region of space around the nucleus. This is where the concept of orbitals comes in. Orbitals are regions of space where there is a high probability of finding an electron.
The shape of an orbital depends on the energy level and the type of orbital (s, p, d, f). For example, the s orbitals are spherical, the p orbitals are dumbbell-shaped, and the d orbitals are more complex.
Understanding these energy levels and orbitals is crucial for understanding how atoms interact with each other and form molecules.
What is the path of an electron around the nucleus?
Think of it like this: Imagine the nucleus as the sun and the electron shells as different-sized rings around the sun. The electrons don’t stay in one place in the rings, they move around quickly, but they are more likely to be found in certain areas within the ring.
It’s important to note that the number of electrons that can fit in a shell is limited. The maximum number of electrons that can occupy a shell is determined by the formula 2n2, where n is the shell number. For example, the first shell, K, has a maximum of 2 electrons (2 x 12 = 2), while the second shell, L, has a maximum of 8 electrons (2 x 22 = 8).
The electron shells are also divided into subshells which are further subdivided into orbitals. Orbitals are regions of space where there is a high probability of finding an electron. Each orbital can hold a maximum of two electrons.
We can see that the simple image of electrons orbiting the nucleus like planets is not completely accurate. The movement of electrons is much more complex and is best described using the quantum mechanical model. This model uses mathematical equations to describe the behavior of electrons in atoms.
So, while the term “orbit” is sometimes used, it’s important to understand that electrons don’t travel in fixed paths. Instead, their behavior is more accurately described as occupying a region of space called a shell.
What are the areas called where electrons travel around the nucleus?
Think of it like this: Imagine a tiny planet orbiting a star. We can’t pinpoint the planet’s exact location at any given moment, but we can say that it’s most likely to be found within a certain region around the star. This region is the planet’s orbit, and in the case of electrons, it’s their electron shell.
Each energy level has a specific amount of energy associated with it. Electrons in the lower energy levels are closer to the nucleus and have lower energy than those in higher energy levels. Electrons can jump between energy levels by absorbing or releasing energy.
For example, if an electron absorbs energy from light, it can jump to a higher energy level. Conversely, if an electron releases energy, it can drop to a lower energy level. This process is what causes atoms to emit light, which is why we see the beautiful colors in fireworks and neon signs.
So, the next time you think about atoms, remember that those tiny particles, the electrons, are constantly buzzing around the nucleus in a fascinating dance.
Where do electrons exist as they orbit the nucleus?
To understand this better, picture a wave on a string tied at both ends. This wave has to fit perfectly between the two ends, creating what we call a standing wave. Electrons behave similarly, but instead of being confined to a string, they are confined to the space around the nucleus. These standing waves are called orbitals. Each orbital has a specific shape and energy level. The lowest energy level is called the ground state, and the higher energy levels are called excited states.
Think of these orbitals like different “parking spots” for electrons around the nucleus. Each orbital can hold a maximum of two electrons. When an electron gains energy, it can “jump” to a higher energy orbital, much like a car moving to a different parking spot. When it loses energy, it “falls” back to a lower energy orbital. This movement between orbitals is what causes electrons to absorb or emit light.
What is the placement of electrons in an atom?
Think of it like this: Imagine a stadium with different seating levels. The lower levels are closer to the field and filled first, just as the inner shells of an atom are filled first with electrons.
Electrons occupy the lowest energy shells available before moving to higher energy shells. They won’t jump to a higher shell until the lower shell is completely filled. This is why you’ll see patterns in the arrangement of electrons in different elements, which is determined by the atom’s atomic number.
Let’s dive a little deeper into how electrons are arranged in these shells. The arrangement of electrons in an atom is described by a model called the electronic configuration, which helps us understand how electrons are distributed within the atom. Here’s how it works:
Principal Quantum Number (n): This number represents the energy level of the electron shell. It can be any positive integer, like 1, 2, 3, and so on. Higher numbers indicate higher energy levels.
Subshells: Within each shell, there are subshells with slightly different energy levels. These subshells are labeled as s, p, d, and f, with s being the lowest energy subshell and f the highest.
Orbitals: Each subshell contains one or more orbitals, which are regions of space where there is a high probability of finding an electron. Each orbital can hold a maximum of two electrons.
So, you can see that the electronic configuration of an atom is a complex but organized system, much like a carefully designed stadium!
Here’s an example: In oxygen, which has an atomic number of 8, the first two electrons occupy the 1s subshell. Then, the next two electrons occupy the 2s subshell. The remaining four electrons fill the 2p subshell. This configuration can be written as 1s² 2s² 2p⁴.
Knowing the electronic configuration of an atom is essential for understanding its chemical behavior, as the arrangement of electrons plays a crucial role in how atoms interact with each other to form bonds and molecules.
See more here: Where Are Electrons Arranged Around The Nucleus? | Where Are Electrons Revolving Around The Nucleus Placed
Where are electrons located?
Think of it like this: the nucleus is like the sun, and the electrons are like planets, but they don’t have fixed orbits like planets do. Instead, they zoom around the nucleus in a cloud-like region.
We can’t pinpoint exactly where an electron is at any given moment, it’s more like a probability game. Scientists use probability distributions to predict where an electron is most likely to be found within the electron cloud.
Here’s a more detailed explanation of the electron cloud:
Imagine a foggy morning. You can’t see through the fog to pinpoint the exact location of each water droplet, right? But you know the fog is there, and you can guess where the droplets are most likely to be found. That’s similar to how we think about electrons in the electron cloud. We can’t know their exact location at any given moment, but we can predict where they’re most likely to be.
There are different regions within the electron cloud, called electron shells, where electrons are likely to be found. These shells are like different energy levels for the electrons. The closer a shell is to the nucleus, the lower its energy level. Electrons can jump between shells by absorbing or releasing energy. This is how atoms interact with each other and how things like light and heat are produced.
So, while we can’t know the exact location of an electron at any one time, we understand how they behave within the electron cloud. This understanding is crucial for understanding the building blocks of the universe and how everything around us works!
How do you find an electron around a nucleus?
Think of it like this: imagine you’re trying to find your cat in a big house. You don’t know exactly where it is, but you can make a guess based on where you’ve seen it before, right? The wave function works like that. It tells us the probability of finding an electron in a certain spot around the nucleus.
Schrödinger’s equation, a very important equation in quantum mechanics, helps us figure out this wave function. It’s a bit like a magic formula that takes into account the forces involved and gives us the most likely places to find the electron.
And just like with your cat, the electron doesn’t have to be in one place. It could be anywhere around the nucleus, but some spots are more likely than others. This probability is what we use to understand how electrons behave around a nucleus.
So, how do we find the electron? We don’t *actually* find it, not in the traditional sense. We can’t point to a specific location and say, “There it is!” Instead, we use the wave function to describe its probability of being in different places, and that’s how we understand its behavior.
What happens when electrons move around the nucleus?
Now, according to James Clerk Maxwell’s work, an accelerating charged object emits radiation. This is a fundamental concept in physics.
But wait, what does this mean for the electrons orbiting the nucleus? Well, if the electrons were truly orbiting the nucleus in a classical sense, like planets orbiting the sun, they would constantly lose energy and spiral inwards towards the nucleus until they collided. This is because the electrons are constantly radiating energy.
This presents a bit of a problem. If electrons are constantly losing energy, atoms shouldn’t be stable!
But, we know that atoms are stable. This means that electrons don’t behave like tiny planets orbiting a star. The classical model of an atom, where electrons orbit the nucleus like planets, doesn’t accurately reflect what’s happening.
So, what’s the real picture? Well, the classical model of the atom had to be revised. The quantum mechanical model of the atom, which is more accurate, says that electrons don’t orbit the nucleus like planets, but rather they exist in orbitals.
Orbitals are regions of space around the nucleus where the probability of finding an electron is high. They are not fixed orbits like the orbits of planets. Instead, they are more like “clouds” of probability, defined by their shape and energy level.
According to the quantum mechanical model, electrons can jump between different orbitals, absorbing or releasing energy in the process. This explains why atoms can absorb and emit light, leading to the phenomenon of spectroscopy.
In summary, the classical model of electrons orbiting the nucleus fails to explain why atoms are stable. The quantum mechanical model provides a more accurate description of the atom, where electrons exist in orbitals and can jump between them, absorbing or releasing energy.
How do you find the position of an electron?
Think of it like this: Imagine you’re throwing a bunch of darts at a target. You can’t predict where any single dart will land, but you can see a pattern emerge—more darts hit the center than the edges. The electron’s wavefunction is like that pattern of darts. It tells us the probability of finding the electron in different areas around the nucleus.
This cloud isn’t a fuzzy, solid sphere, but rather a probability map. It represents the most likely regions where the electron exists. In simple terms, the electron’s position is not fixed, but rather exists as a probability distribution around the nucleus. The shape of this probability distribution is determined by the energy level of the electron.
Let’s break down the concepts a bit more:
Wavefunction: The wavefunction is a mathematical function that describes the behavior of an electron in an atom. It’s not a physical thing you can see or touch, but a mathematical tool that helps us understand the electron’s properties.
Probability Distribution: The probability distribution is a visual representation of where the electron is likely to be found. It’s like a heat map, where the brighter areas represent a higher probability of finding the electron.
Quantum Mechanics: The idea that we can only know the probability of finding an electron in a specific location is a fundamental concept in quantum mechanics. It’s a strange and counterintuitive concept, but it’s the best way we have to understand the behavior of electrons and other tiny particles.
In summary, we can’t know an electron’s exact position. Instead, we use the wavefunction to describe its probability distribution, which is represented as a cloud around the nucleus. This cloud shows us the areas where the electron is most likely to be found.
See more new information: linksofstrathaven.com
Where Are Electrons Revolving Around The Nucleus Placed | Where Do Electrons Move Around The Nucleus?
Electrons Don’t Just Orbit: They’re in Orbitals
Picture it: a tiny, dense nucleus in the center of the atom, and buzzing around it are these super-fast electrons. But they don’t just orbit like planets around the sun. Instead, they exist in these areas of space called orbitals.
Think of orbitals as probability clouds. It’s like saying, “We’re not sure where the electron is exactly, but there’s a higher chance of finding it in this particular region.” These orbitals have different shapes and sizes, and each can hold a maximum of two electrons.
The Atomic Model: From Bohr to Quantum Mechanics
You’ve probably seen those diagrams with the nucleus in the center and electrons in circular orbits. That’s the Bohr model. It’s simple, but it’s not exactly what’s going on.
Bohr’s model helped us understand some basic things about atoms, but it didn’t account for the weirdness of the quantum world. You know, things like wave-particle duality where electrons act like both waves and particles.
We needed a new model, and that’s where quantum mechanics came in. It uses wave functions to describe the probability of finding an electron in a particular region of space.
What are These Orbitals, Anyway?
Here’s where things get interesting. Orbitals aren’t just random shapes. They’re defined by quantum numbers, which basically give each electron a unique address within the atom. There are four main types:
Principal Quantum Number (n): This describes the electron’s energy level. The higher the number, the farther from the nucleus it is and the more energy it has. Think of it like the “floor” of an apartment building.
Angular Momentum Quantum Number (l): This describes the shape of the orbital. It can be s, p, d, or f (with s being spherical and the others getting more complex). You can think of this as the “room” within the apartment.
Magnetic Quantum Number (ml): This describes the orientation of the orbital in space. It basically tells us where the room is pointing.
Spin Quantum Number (ms): This describes the intrinsic angular momentum of an electron, which we can think of as its spin.
How Do We Know All This?
We can’t just see these orbitals directly. Scientists use spectroscopy to analyze the light emitted or absorbed by atoms. This tells us what energy levels the electrons are in and helps us figure out the orbital shapes. It’s like a fingerprint of the atom!
Where Are Electrons in an Atom?
So, to finally answer your question, electrons aren’t spinning around the nucleus in perfect circles. They’re in orbitals, which are regions of space where there’s a high probability of finding an electron.
s Orbitals are spherical. So, there’s an equal chance of finding the electron at any point on the sphere.
p Orbitals have a dumbbell shape with two lobes on either side of the nucleus.
d Orbitals are even more complex and have various shapes, like cloverleafs and double dumbbells.
f Orbitals are the most complicated and have even stranger shapes.
The principal quantum number (n) tells us which shell (or energy level) an orbital is in. So, the 1s orbital is the lowest energy level, the 2s is the next, and so on.
Remember: the higher the energy level, the farther the electron is from the nucleus, on average.
Key Takeaways:
* Electrons don’t simply orbit the nucleus.
* They occupy orbitals, which are probability clouds.
Quantum mechanics describes the behavior of electrons in atoms.
Quantum numbers define the energy levels, shapes, and orientations of orbitals.
Spectroscopy is a powerful tool for studying the structure of atoms.
Frequently Asked Questions (FAQs)
1. How many electrons can fit in an orbital?
Only two electrons can occupy a single orbital. This is due to the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers.
2. What is the difference between an orbit and an orbital?
A orbit is a circular path that an object follows around another object, like a planet around a star. An orbital is a three-dimensional region of space where there is a high probability of finding an electron.
3. Can electrons jump between orbitals?
Yes! Electrons can jump between orbitals by absorbing or emitting energy in the form of light. This is called an electronic transition. For example, if an electron absorbs enough energy, it can jump from a lower energy level to a higher one.
4. Why do electrons have different energy levels?
The energy level of an electron depends on its distance from the nucleus and the strength of the attraction between the electron and the nucleus. The closer an electron is to the nucleus, the more strongly it is attracted, and the lower its energy level.
5. What is the significance of quantum mechanics?
Quantum mechanics revolutionized our understanding of atoms and molecules. It explains why certain materials are conductors, semiconductors, or insulators. It also helps us to understand the behavior of light, lasers, and transistors, which are crucial for modern technology.
6. Is this all there is to know about the structure of atoms?
Not quite! There are many other aspects to explore, like molecular orbitals which describe the behavior of electrons in molecules, and electron configuration, which describes the arrangement of electrons in different orbitals.
7. How do we visualize orbitals?
We use computer models and simulations to visualize orbitals. They’re not perfect representations, but they help us to understand the shapes and relative sizes of these probability clouds.
8. What are some real-world applications of this knowledge?
The study of atomic structure is fundamental to many fields, including chemistry, materials science, medicine, and nuclear physics. It allows us to develop new materials, understand chemical reactions, and even design new drugs.
9. Is the model we use to describe electrons perfect?
No model is perfect. Even our current understanding of quantum mechanics is constantly evolving and being refined. It’s a journey of discovery and exploration.
10. How can I learn more?
There are many resources available to help you learn more about atomic structure. You can check out textbooks, online courses, or even just search for videos and articles on the topic.
Remember, understanding the structure of atoms is essential for understanding the world around us. Keep exploring and asking questions!
Where do electrons get energy to spin around an
An electron orbiting a nucleus is electrically attracted to the nucleus; it’s always being pulled closer. But the electron also has kinetic energy, which works to send the electron flying away. Live Science
The movement of electrons around the nucleus and
The electrons revolve around the nucleus in a number of orbits called the energy levels, and during their movement, they Science online
How does electron move around nucleus? – Physics Stack
Electron around nucleus is described by complex wave function with both real and imaginary parts.The electron is no longer described as moving around the nucleus but Physics Stack Exchange
Where do electrons get their ever-lasting circulating energy?
the position of the electron is described by it’s wavefunction, and that gives you a ‘cloud’ around the nucleus with the probability distribution of where you will find the electron. Physics Stack Exchange
Why do electrons not fall into the nucleus?
A revolving electron would transform the atom into a miniature radio station, the energy output of which would be at the cost of the potential energy of the electron; according to classical Chemistry LibreTexts
Chapter 2: Electrons and Orbitals – Chemistry LibreTexts
Assuming that the electrons are moving around the nucleus, they are constantly accelerating (changing direction). If you know your physics, you will recognize that (as Chemistry LibreTexts
Where Are the Electrons Located in an Atom? – Science Notes
Electrons are located in the electron cloud, which surrounds the nucleus of an atom. This electron cloud represents regions where electrons are likely to be found, Science Notes and Projects
Why do electrons revolve around the nucleus?
Why do electrons revolve around the atom’s nucleus? The first approximate planetary model, Bohr model, is discussed in G.Smith’s answer. There the electron is caught at the lowest energy bound Physics Stack Exchange
Questions and Answers – What keeps the electrons revolving
What keeps the electrons revolving around the nucleus of an atom? This question goes right to the heart of why quantum mechanics became the model for describing what TJNAF Educational Programs
Inquiring Minds – Questions About Physics
Why does the electron have to move around the Nucleus? Why does it not lose energy moving around it? The answer is: although it is convenient to think of the electron Fermilab
Where Do Electrons Get Energy To Spin Around An Atom’S Nucleus?
Where Do Electrons Get Their Everlasting Energy?
How Electrons Orbiting The Nucleus Never Fall Into The Nucleus?
Atom 3D Model Orbital Spin
The Uncertain Location Of Electrons – George Zaidan And Charles Morton
How Does Electron Move Around Nucleus? Gryzinski’S Free-Fall Atomic Models For Chemical Elements
Why Don’T Electrons Fall Into The Nucleus| Quantum Mechanics| Breakthrough Junior Challenge 2022
Link to this article: where are electrons revolving around the nucleus placed.
See more articles in the same category here: https://linksofstrathaven.com/how
