What is the electron configuration of a neutral beryllium atom?
1s²2s² represents the arrangement of electrons in a neutral beryllium atom. This configuration signifies that the 1s orbital is completely filled, containing two electrons, while the 2s subshell holds the remaining two electrons in a paired state.
To understand this better, let’s delve deeper. The electron configuration tells us where electrons reside within an atom’s energy levels and sublevels.
Beryllium (Be), with an atomic number of 4, has four electrons. These electrons occupy specific energy levels and sublevels, dictated by the Aufbau principle and Hund’s rule.
The Aufbau principle states that electrons occupy the lowest available energy levels first. The 1s orbital is the lowest energy level, so it fills first with two electrons. Next, the 2s orbital, being the next lowest energy level, receives the remaining two electrons.
Hund’s rule, on the other hand, states that electrons prefer to occupy different orbitals within a subshell, with parallel spins, before pairing up in the same orbital. In the case of beryllium, the 2s subshell has only one orbital, so the two electrons pair up within this orbital.
Therefore, 1s²2s² accurately depicts the electron configuration of a neutral beryllium atom, reflecting the principles that govern electron arrangement.
What is the neutral electron configuration of an atom?
1s22s22p63s23p2 is a specific example of an electron configuration, and it represents the element silicon (Si).
But what exactly does this configuration mean?
Think of it like this: the electron configuration of an atom describes the arrangement of its electrons in different energy levels. These levels are represented by numbers (1, 2, 3, etc.), and within each level, there are sublevels (s, p, d, and f).
The superscripts in the configuration tell us how many electrons are in each sublevel. So, in the case of silicon, we have:
1s2: Two electrons in the first energy level, specifically in the ‘s’ sublevel.
2s2: Two electrons in the second energy level, in the ‘s’ sublevel.
2p6: Six electrons in the second energy level, in the ‘p’ sublevel.
3s2: Two electrons in the third energy level, in the ‘s’ sublevel.
3p2: Two electrons in the third energy level, in the ‘p’ sublevel.
Now, why is this configuration specific to a neutral silicon atom? The number of electrons in an atom determines its overall charge. A neutral atom has an equal number of protons (positively charged particles) and electrons (negatively charged particles). Since silicon has 14 protons, a neutral silicon atom will also have 14 electrons, leading to this particular configuration.
Remember that every element has a unique electron configuration that reflects its atomic structure. Understanding these configurations helps us understand the chemical behavior and properties of different elements.
What is the symbol for neutral beryllium atom?
Now, let’s talk about the neutral beryllium atom. This is an atom of beryllium that has an equal number of protons and electrons. Since it has four protons (positive charge), it also has four electrons (negative charge) orbiting the nucleus. The equal number of positive and negative charges makes the atom electrically neutral, hence the name.
This neutrality is important because it means beryllium atoms don’t have a strong tendency to gain or lose electrons. This makes them pretty stable and less likely to react with other elements. That’s why beryllium is often used in alloys, where its strength and lightness are valuable properties.
To summarize, the symbol for a neutral beryllium atom is Be. This symbol represents the element itself, while the fact that it’s neutral indicates that it has an equal number of protons and electrons.
How to write electron configuration?
You’re right, there’s a specific way to write them, and it helps us understand how electrons are arranged within an atom. It’s like a special code!
Here’s the basic format:
First, you write the energy level. This tells us how far from the nucleus the electrons are. Think of it like the floors in a building, with lower numbers indicating closer proximity to the nucleus.
Next, you write the type of orbital. There are four main types: s, p, d, and f. They have different shapes and hold a specific number of electrons:
s orbitals are spherical and hold a maximum of 2 electrons.
p orbitals are dumbbell-shaped and can hold a maximum of 6 electrons.
d orbitals are more complex in shape and can hold a maximum of 10 electrons.
f orbitals are even more complicated and can hold a maximum of 14 electrons.
Finally, you write the number of electrons in that orbital as a superscript. For example, 2s2 means there are two electrons in the 2s orbital.
Let’s break down the example of carbon (atomic number 6):
1s2: This means there are two electrons in the 1s orbital, which is the lowest energy level.
2s2: There are two electrons in the 2s orbital of the second energy level.
2p2: Two electrons reside in the 2p orbitals of the second energy level.
To summarize, the electron configuration of carbon is 1s22s22p2.
Remember, the order of the orbitals in the electron configuration follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This means that 1s is always filled before 2s, and 2s is filled before 2p.
Now, you can use this same method to write the electron configurations for other elements. Just remember to follow the Aufbau principle and the maximum electron capacity of each orbital!
How do you make a neutral beryllium atom?
Beryllium has an atomic number of 4, which means it has 4 protons. The number of protons determines what element it is. Beryllium also has a mass number of about 9 amu, which tells us the total number of protons and neutrons in the atom. So, we know that beryllium has 5 neutrons.
Now, here’s the key to making a neutral beryllium atom: we need an equal number of protons and electrons. Since beryllium has 4 protons, it also needs 4 electrons to be neutral.
Think of it like this: protons have a positive charge, and electrons have a negative charge. If they’re balanced, the atom is neutral!
So, a neutral beryllium atom is made up of 4 protons, 4 electrons, and 5 neutrons.
Let’s dig a little deeper into how we get a neutral beryllium atom. You can’t really “make” one from scratch! We find beryllium naturally occurring in the environment. It’s actually quite rare! Beryllium atoms are found in rocks, soil, and even some plants.
Here’s the cool thing: when scientists talk about “making” an atom neutral, they’re usually referring to situations where the atom has gained or lost electrons. Think about a beryllium atom that has lost one or more of its electrons. Now it’s no longer neutral, right? It has more protons than electrons. We call this a beryllium ion. To make this ion neutral again, we need to add electrons back to it. This is how we “make” a neutral beryllium atom, by restoring the balance between protons and electrons.
The good news is that nature is pretty good at balancing things out! So, in most cases, beryllium atoms in the environment are already in their neutral state.
What is the electron configuration of a neutral atom of sulfur?
Let’s break down what this configuration means. The numbers, like 1 and 2, represent the energy levels or shells where the electrons are located. The letters, s and p, refer to the subshells within those energy levels. The superscript numbers, like 2 and 6, indicate the number of electrons in each subshell.
For sulfur, the 1s2 part means there are two electrons in the first energy level (n=1) in the s subshell. Similarly, 2s2 means there are two electrons in the s subshell of the second energy level (n=2). The 2p6 tells us that there are six electrons in the p subshell of the second energy level. Moving to the third energy level (n=3), we have 3s2 with two electrons in the s subshell and 3p4 with four electrons in the p subshell.
The p subshell can hold a maximum of six electrons. Each p subshell contains three orbitals, and each orbital can hold a maximum of two electrons. This means sulfur has two fully filled p orbitals and one partially filled p orbital. This partially filled orbital is what makes sulfur reactive and capable of forming bonds with other elements.
See more here: What Is The Neutral Atom Of Beryllium Atomic Number? | Write The Electron Configuration For A Neutral Atom Of Beryllium.
What is the electron configuration of beryllium?
The electron configuration of beryllium is [He] 2s². This means that the first two electrons fill the 1s orbital, just like helium. The remaining two electrons are in the 2s orbital.
Beryllium is a light, strong metal that’s silvery-gray in color. It’s found in a variety of minerals, like beryl, and is used in alloys to make them stronger. It’s also used in nuclear reactors and in electronics.
You might be wondering why beryllium has such a simple electron configuration. Well, it’s all about its position on the periodic table! Beryllium is in the second period, which means that its electrons only occupy the first two energy levels. The first energy level only has the 1s orbital, which holds up to two electrons. The second energy level has the 2s and 2p orbitals, but beryllium only has enough electrons to fill the 2s orbital.
This simple electron configuration is one of the things that makes beryllium so interesting. It also explains why beryllium is so reactive. Because it only has two electrons in its outer shell, it easily loses them to form chemical bonds with other atoms.
Remember, electron configurations are super important for understanding how elements behave. They help us understand the reactivity, bonding, and properties of all the elements on the periodic table!
How do you write an electron configuration?
When writing an electron configuration, you follow a specific order. The ground-state electron configuration describes the arrangement of electrons in an atom in its most stable state. This arrangement is based on filling the orbitals in a specific order, following the Aufbau principle. For instance, the ground-state electron configuration of beryllium is 1s² 2s². This means that beryllium has two electrons in its 1s orbital and two electrons in its 2s orbital.
When an atom absorbs energy, it can transition to an excited state. In this state, an electron jumps to a higher energy level, causing a change in the electron configuration. We can represent this change by moving an electron from a lower energy level to a higher energy level. For example, if we excite a beryllium atom, one of the 2s electrons might jump to the 2p orbital. This would result in an excited-state configuration of 1s² 2s¹ 2p¹.
Here’s a more detailed explanation of how to write an electron configuration:
1. Start with the lowest energy level: Begin by filling the 1s orbital with up to two electrons.
2. Move to the next energy level: After the 1s orbital, you move to the 2s orbital, again filling it with up to two electrons.
3. Follow the Aufbau principle: The Aufbau principle states that you fill the orbitals in order of increasing energy. This means that you would fill the 2p orbitals after the 2s orbital. The 2p orbitals can hold up to six electrons.
4. Continue filling orbitals: Continue filling the orbitals in this order: 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
Here are some additional things to keep in mind:
Orbitals have specific capacities: Each orbital can hold a maximum number of electrons:
* s orbitals can hold up to two electrons.
* p orbitals can hold up to six electrons.
* d orbitals can hold up to ten electrons.
* f orbitals can hold up to fourteen electrons.
Use superscripts to indicate the number of electrons: The superscript following the orbital name tells you how many electrons are in that orbital.
Let’s practice with another example:
Nitrogen (N) has an atomic number of 7. This means it has 7 electrons. We can write its electron configuration as 1s² 2s² 2p³.
Remember: Writing electron configurations is a fundamental skill in chemistry. By understanding how to determine electron configurations, you can better understand the behavior of atoms and the bonds they form.
Is beryllium a cation element?
A beryllium atom readily donates its two outer shell electrons to form a beryllium ion, Be2+. This process leaves beryllium with a positive charge, and since it forms a bond by donating electrons, it is considered a cation. The electron configuration of Be2+ is 1s2.
Now, let’s dive a bit deeper into what makes beryllium so eager to give up those electrons.
Beryllium is a small atom with a relatively high ionization energy. This means that it takes a fair amount of energy to remove an electron from a beryllium atom. However, once you remove one electron, the beryllium atom becomes a beryllium ion with a positive charge. This positive charge makes it much easier to remove the second electron, as the remaining electrons are more strongly attracted to the now positively charged nucleus. This two-electron donation pattern is common for beryllium in chemical reactions, making it a reliable source of positive charge and a crucial element in various compounds and materials.
Think of it like this: beryllium wants to be stable, and achieving a full outer shell of electrons is the key. By giving up its two outer electrons, beryllium achieves a stable, filled inner shell, mimicking the noble gas configuration of helium. This makes it a happy, stable cation, ready to bond with other elements and form new compounds.
What is the atomic number of a beryllium atom?
These electrons are arranged in shells around the nucleus. The first shell can hold up to two electrons, and the second shell can hold up to eight. Therefore, a beryllium atom will have two electrons in the first shell and two in the second shell, making the electron configuration 2, 2.
This specific arrangement of electrons in shells is important because it determines how beryllium interacts with other elements. Beryllium is a small, lightweight element with a strong affinity for forming bonds with other elements. This makes it a valuable component in many different materials, including alloys, ceramics, and semiconductors.
Beryllium is a relatively rare element, but it’s found in many different parts of the world. It’s also an important part of the Earth’s crust. It’s found in many different minerals, and it’s even used in some nuclear power plants.
Beryllium is a very interesting element with a lot of unique properties. I hope this helps you understand it better.
See more new information: linksofstrathaven.com
Write The Electron Configuration For A Neutral Atom Of Beryllium: A Step-By-Step Guide
Electron Configuration of Beryllium
We know beryllium (Be) is a small, shiny metal, and like all elements, it has a unique arrangement of electrons. Its electron configuration tells us exactly how those electrons are distributed within its energy levels and orbitals.
Here’s how we find it:
1. Find Beryllium’s Atomic Number: You’ll find it on the periodic table, it’s 4. This number represents the total number of protons in beryllium’s nucleus, and since it’s a neutral atom, it also tells us the number of electrons.
2. Use the Aufbau Principle: This principle helps us fill the orbitals in order of increasing energy. We start with the lowest energy level and move up.
3. Follow Hund’s Rule: This rule states that we fill each orbital within a subshell individually before doubling up in any one orbital.
4. Apply the Pauli Exclusion Principle: This principle states that no two electrons can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, and those electrons must have opposite spins.
Now let’s do it for beryllium:
1s² 2s²
Let’s break it down:
1s²: This part tells us that the first energy level (n=1) has a subshell called *s*, and it’s filled with two electrons. The superscript “2” indicates the number of electrons in the *s* orbital.
2s²: This part tells us that the second energy level (n=2) also has an *s* subshell, and it too is filled with two electrons.
So, there you have it, the electron configuration of beryllium is 1s² 2s².
A Visual Representation
Here’s how you can visualize this configuration:
First Energy Level (n=1):
* One *s* orbital (holds up to 2 electrons)
* Filled with 2 electrons
Second Energy Level (n=2):
* One *s* orbital (holds up to 2 electrons)
* Filled with 2 electrons
Important Notes:
Valence Electrons: The electrons in the outermost energy level are called valence electrons. In beryllium’s case, the valence electrons are in the 2s² orbital. They are the ones that participate in chemical bonding and determine the element’s reactivity.
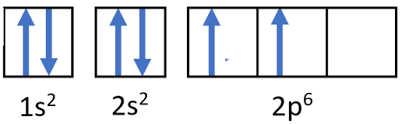
Orbital Diagram: An orbital diagram is a visual representation of the electron configuration, showing each orbital as a box and the electrons as arrows. Here’s what the orbital diagram for beryllium would look like:
“`
1s: ↑↓
2s: ↑↓
“`
Why Does This Matter?
Understanding the electron configuration of beryllium is key to comprehending its chemical behavior. This configuration explains why it’s a good conductor of heat and electricity, its relatively low melting point, and its tendency to form ionic bonds.
FAQs
1. What are the quantum numbers for the electrons in beryllium?
* We know that beryllium has 4 electrons, so we can assign the quantum numbers for each electron:
Electron 1: (n=1, l=0, ml=0, s=+1/2) This electron is in the 1s orbital with spin up.
Electron 2: (n=1, l=0, ml=0, s=-1/2) This electron is in the 1s orbital with spin down.
Electron 3: (n=2, l=0, ml=0, s=+1/2) This electron is in the 2s orbital with spin up.
Electron 4: (n=2, l=0, ml=0, s=-1/2) This electron is in the 2s orbital with spin down.
2. How do I write the electron configuration of an excited state of beryllium?
* An excited state occurs when an electron absorbs energy and jumps to a higher energy level. For example, an excited state of beryllium could have the configuration 1s² 2s¹ 2p¹
3. How does the electron configuration relate to beryllium’s position on the periodic table?
* The periodic table is organized based on electron configuration. Beryllium is in the second period, which means it has two electron shells. It’s in Group 2 (alkaline earth metals), which means it has two valence electrons, as reflected in its 2s² configuration.
4. What is the difference between the electron configuration and the orbital diagram?
* The electron configuration is a shorthand way to represent the distribution of electrons in an atom. It shows the energy levels and subshells, but doesn’t explicitly show the individual orbitals.
* The orbital diagram is a visual representation that shows each orbital as a box and the electrons as arrows. It helps visualize how the electrons are arranged within the orbitals.
Key Takeaways
* Beryllium’s electron configuration is 1s² 2s².
* This configuration tells us how the electrons are arranged in its energy levels and orbitals.
* The electron configuration of an atom is essential for understanding its chemical properties and how it bonds with other atoms.
I hope you found this explanation helpful! Let me know if you have any other questions about beryllium or electron configurations.
How to Write the Electron Configuration for Beryllium
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. TerpConnect
How To Write The Electron Configuration For A Neutral Atom Of
The electron configuration of a neutral atom of beryllium is written as 1s²2s². The Aufbau Principle, Hund’s Rule, and the Pauli-Exclusion Principle determine the electron All About Metals
Electron configuration of Beryllium
Electron configuration of Beryllium. The electron configuration of beryllium is [He] 2s2. Beryllium is defined as a chemical element whose symbol is Be, its atomic number is 4. Electron Configuration
Solved Write the electron configuration for a neutral atom – Chegg
Write the electron configuration for a neutral atom of beryllium Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you Chegg
Electron Configuration Calculator
Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Omni Calculator
6.8: Electron Configurations – Chemistry LibreTexts
For hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (Figure \(\PageIndex{1}\)), and the electron configuration is written Chemistry LibreTexts
Beryllium Electron Configuration:Everything You Need to Know
How to write Beryllium electron configuration? The electron configuration of Be is written with the help of shell number, subshell number, and three basic principles techiescience.com
3.1: Electron Configurations – Chemistry LibreTexts
First, write the electron configuration for the neutral atoms: Zn: [Ar]3d 10 4s 2; Cr: [Ar]3d 5 4s 1; Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from Chemistry LibreTexts
Electron configurations article (article) | Khan Academy
Microsoft Teams. What are electron configurations? The cells in our bodies are masters of quantum physics—they’ve figured out the complicated dance of atoms and electrons, Khan Academy
Beryllium Electron Configuration
Aleks: Writing The Electron Configuration Of A Neutral Atom With A And P Electrons Only
Aleks: Drawing A Box Diagram Of The Electron Configuration Of An Atom
Be 2+ Electron Configuration (Beryllium Ion)
Aleks: Interpreting The Electron Configuration Of A Neutral Atom
Electron Configuration – Basic Introduction
Aleks: Writing The Electron Configuration Of A Neutral Atom With A Filled D Subshell
Link to this article: write the electron configuration for a neutral atom of beryllium..
See more articles in the same category here: https://linksofstrathaven.com/how