Let’s discuss the question: 3na2so4 how many molecules. We summarize all relevant answers in section Q&A of website Linksofstrathaven.com in category: Blog Finance. See more related questions in the comments below.
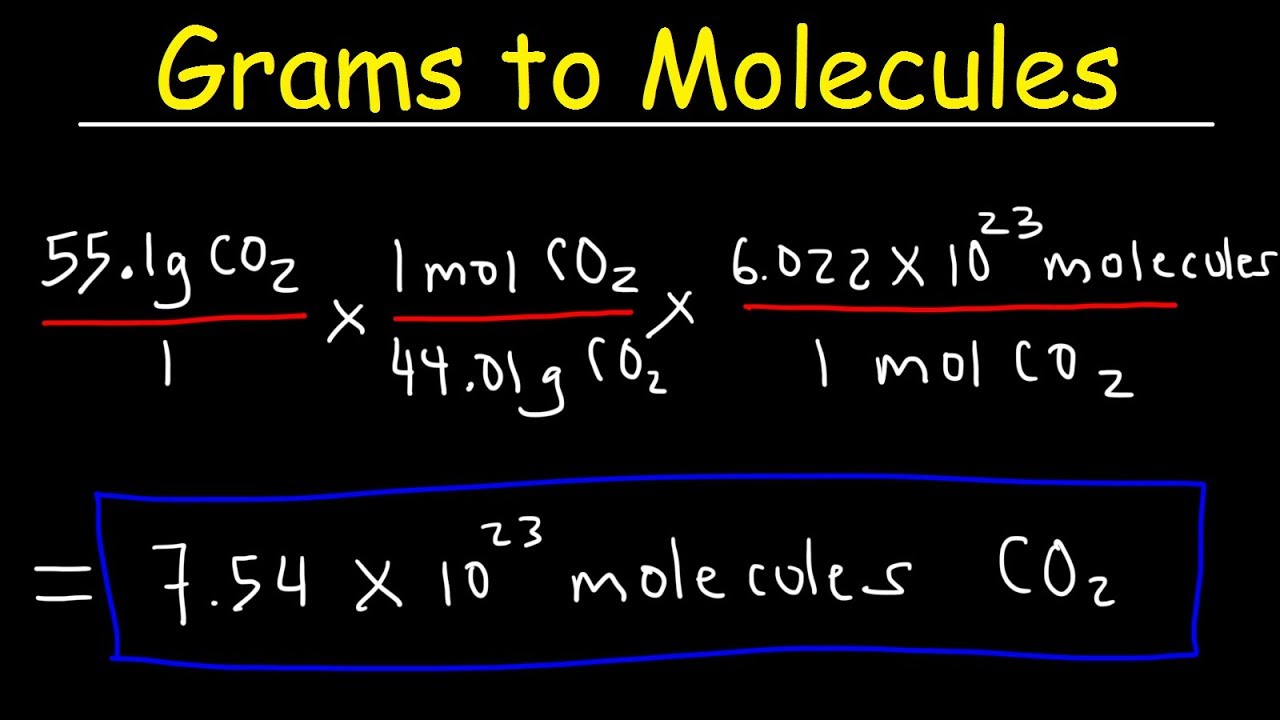
Table of Contents
How many atoms does znso4?
It has one zinc atom, four oxygen atoms a one sulfur atom.
How many molecules are in a molecule?
Molecule: group of two or more atoms held together by chemical bonds. So, minimum 2 atoms are required to form a molecule.
Grams to Molecules and Molecules to Grams Conversion
Images related to the topicGrams to Molecules and Molecules to Grams Conversion

How many molecules are in 1.5 moles?
Solution. Hence, number of molecules in 1.5 moles of ammonia is 9.033 × 1023.
What is molecule number?
To calculate the number of molecules in a substance
The total number of atoms/molecules in a sample can be calculated by multiplying the number of moles with the Avogadro constant. This formula can be written as: Number of Atoms or Molecules = (Number of Moles)*(6.022*1023)
What are the elements of ZnSO4?
It contains a zinc(2+). Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as “white vitriol”.
How many compounds and atoms are in the Formula 5 ZnSO4?
| Element | Symbol | # of Atoms |
|---|---|---|
| Zinc | Zn | 1 |
| Oxygen | O | 4 |
| Sulfur | S | 1 |
How many zinc atoms are in ZnSO4?
The number of atoms of each element present is: Zinc (1 atom) Sulfur (1atom) Oxygen (4 atoms)
How many molecules are in a cell?
University of Toronto. “A cell holds 42 million protein molecules, scientists reveal.” ScienceDaily. ScienceDaily, 17 January 2018.
How many atoms are there in molecules?
A molecule is a group of two or more atoms held together by chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion.
How many atoms make molecules?
molecule, a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance.
How to find the Number of Atoms in a Molecule
Images related to the topicHow to find the Number of Atoms in a Molecule

How many molecules are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
How many oxygen atoms are in an Al2 CO3 3?
According to formulas posted by Crescent High School, there are nine oxygen atoms in a molecule of Al2(CO3)3, aluminum carbonate, because there are three sets of carbonate, CO3, ions. There are also two aluminum and three carbon atoms in total.
How many molecules are in 2 moles?
If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6H 6 molecules, or 3.011 × 10 23 C 6H 6 molecules.
How many atoms are in 4.5 moles?
1 Expert Answer
A mole of anything has 6.022 x 1023 items in it. 4.5 moles of copper has (4.5)(6.022 x 1023) = 2.7 x 1024 atoms.
How many molecules is 3 moles?
A mole of anything contains 6.022×1023 individual items of that something. You have 3 moles, so there are 3×6.022×1023 oxygen molecules .
How many molecules are in 2 moles of so3?
2 moles of SO3 contain 2 x 6.02 × 10 23 = 12.04 x 10 23 molecules.
How many molecules are there in H2O?
For example: H2O has two hydrogens and one oxygen. The atomic mass of hydrogen is 1.0078 and the atomic mass of oxygen is 15.999, so the mass of a mole of water is 2 x 1.0078 + 15.999. Therefore, a mole of water, or 6.022 x 1023 molecules, weighs 18.0146 grams.
What is number of molecules in 9.0 g of steam?
9 gram of steam contain 3.015*10^23 H2O molecules. Note: 9 gram of water whether as ice or liquid water or steam always contains 3.015*10^23 water molecules. 9 grams of ice or liquid water or steam have different volumes but same number of molecules .
What is the molecular mass of ZnSO4?
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

What is ZnSO4 used for?
Zinc sulfate is a drug used to replenish low levels of zinc or prevent zinc deficiency, or to test for zinc deficiency. Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as “white vitriol”.
How do you identify ZnSO4?
Zinc Sulfate is odourless and has a white powder appearance. Zinc Sulfate is non combustible and is soluble in water.
Related searches
- how many molecules does 3na2so4 have
- how many moles of so4 are in 3na2so4
- 3na2so4 state of matter
- what is the number of molecules in 3na2so4
- 3na2so4 chemical name
- 3na2so4 element
- how many elements are in 3na2so4
- what is the maximum number of molecules
- what is 3na2so4
- total atoms in 3na2so4
- 3na2so4 name of element
- how many molecules are in 2h2o
- number of molecules in 2znso4
- k2co3 number of molecules
Information related to the topic 3na2so4 how many molecules
Here are the search results of the thread 3na2so4 how many molecules from Bing. You can read more if you want.
You have just come across an article on the topic 3na2so4 how many molecules. If you found this article useful, please share it. Thank you very much.
